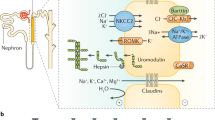
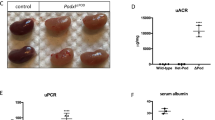
Abstract
Familial idiopathic nephrotic syndromes represent a heterogeneous group of kidney disorders, and include autosomal recessive steroid-resistant nephrotic syndrome, which is characterized by early childhood onset of proteinuria, rapid progression to end-stage renal disease and focal segmental glomerulosclerosis. A causative gene for this disease, NPHS2, was mapped to 1q25–31 and we report here its identification by positional cloning. NPHS2 is almost exclusively expressed in the podocytes of fetal and mature kidney glomeruli, and encodes a new integral membrane protein, podocin, belonging to the stomatin protein family. We found ten different NPHS2 mutations, comprising nonsense, frameshift and missense mutations, to segregate with the disease, demonstrating a crucial role for podocin in the function of the glomerular filtration barrier.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Accession codes
References
Olson, J.L. & Schwartz, M.M. The nephrotic syndrome: minimal change disease, focal segmental glomerulosclerosis, and miscellaneous causes . in Heptinstall's Pathology of the Kidney (eds Jennette, J.C., Olson, J.L. & Silva, F.G.) 187–257 (Lippincott-Raven, Philadelphia, 1998).
Broyer, M., Meyrier, A., Niaudet, P. & Habib, R. Minimal changes and focal segmental glomerular sclerosis. in Oxford Textbook of Clinical Nephrology (eds Davison, A.M. et al.) 493– 535 (Oxford University Press, Oxford, 1998).
Kestilä, M. et al. Positionally cloned gene for a novel glomerular protein—nephrin—is mutated in congenital nephrotic syndrome. Mol. Cell 1, 575–582 (1998).
Ruotsalainen, V. et al. Nephrin is specifically located at the slit diaphragm of glomerular podocytes. Proc. Natl Acad. Sci. USA 96, 7962–7967 (1999).
Holthofer, H. et al. Nephrin localizes at the podocyte filtration slit area and is characteristically spliced in the human kidney. Am. J. Pathol. 155, 1681–1687 ( 1999).
Holzman, L.B. et al. Nephrin localizes to the slit pore of the glomerular epithelial cell. Kidney Int. 56, 1481– 1491 (1999).
Mathis, B.J. et al. A locus for inherited focal segmental glomerulosclerosis maps to chromosome 19q13. Kidney Int. 53, 282 –286 (1998).
Winn, M.P. et al. Linkage of a gene causing familial focal segmental glomerulosclerosis to chromosome 11 and further evidence of genetic heterogeneity. Genomics 58, 113–120 ( 1999).
Fuchshuber, A. et al. Mapping a gene (SRN1) to chromosome 1q25–q31 in idiopathic nephrotic syndrome confirms a distinct entity of autosomal recessive nephrosis. Hum. Mol. Genet. 4, 2155– 2158 (1995).
Dib, C. et al. A comprehensive genetic map of the human genome based on 5,264 microsatellites. Nature 380, 152– 154 (1996).
Kozak, M. Interpreting cDNA sequences: some insights from studies on translation. Mamm. Genome 7, 563–574 (1996).
Prestridge, D.S. Predicting Pol II promoter sequences using transcription factor binding sites. J. Mol. Biol. 249, 923– 932 (1995).
Bairoch, A., Bucher, P. & Hofmann, K. The PROSITE database, its status in 1995. Nucleic Acids Res. 24, 189–196 (1996).
Nakai, K. & Kanehisa, M. A knowledge base for predicting protein localization sites in eukaryotic cells. Genomics 14, 897–911 (1992).
Snyers, L., Umlauf, E. & Prohaska, R. Cysteine 29 is the major palmitoylation site on stomatin. FEBS Lett. 449, 101–104 (1999).
Stewart, G.W. et al. Isolation of cDNA coding for an ubiquitous membrane protein deficient in high Na+, low K+ stomatocytic erythrocytes. Blood 79, 1593–1601 (1992).
Huang, M., Gu, G., Ferguson, E.L. & Chalfie, M. A stomatin-like protein necessary for mechanosensation in C. elegans. Nature 378, 292–295 (1995).
Mannsfeldt, A.G., Carroll, P., Stucky, C.L. & Lewin, G.R. Stomatin, a MEC-2 like protein, is expressed by mammalian sensory neurons. Mol. Cell. Neurosci. 13, 391– 404 (1999).
Salzer, U., Ahorn, H. & Prohaska, R. Identification of the phosphorylation site on human erythrocyte band 7 integral membrane protein: implications for a monotopic protein structure. Biochim. Biophys. Acta. 1151, 149–152 (1993).
Snyers, L., Umlauf, E. & Prohaska, R. Oligomeric nature of the integral membrane protein stomatin. J. Biol. Chem. 273, 17221– 17226 (1998).
Engelman, J.A., Zhang, X.L., Razani, B., Pestell, R.G. & Lisanti, M.P. p42/44 MAP kinase-dependent and -independent signaling pathways regulate caveolin-1 gene expression. J. Biol. Chem. 274, 32333–32341 (1999).
Shih, N.Y. et al. Congenital nephrotic syndrome in mice lacking CD2-associated protein. Science 286, 312– 315 (1999).
Kirsch, K.H., Georgescu, M.M., Ishimaru, S. & Hanafusa, H. CMS: an adapter molecule involved in cytoskeletal rearrangements. Proc. Natl Acad. Sci. USA 96, 6211– 6216 (1999).
Noakes, P.G. et al. The renal glomerulus of mice lacking s-laminin/laminin β2: nephrosis despite molecular compensation by laminin β1. Nature Genet. 10, 400–406 ( 1995).
Parving, H.H. et al. Prevalence and causes of albuminuria in non-insulin-dependent diabetic patients. Kidney Int. 41, 758– 762 (1992).
Connolly, J.O., Weston, C.E. & Hendry, B.M. HIV-associated renal disease in London hospitals. Q. J. Med. 88, 627–634 (1995).
Verani, R.R. Obesity-associated focal segmental glomerulosclerosis: pathological features of the lesion and relationship with cardiomegaly and hyperlipidemia. Am. J. Kidney Dis. 20, 629–634 (1992).
Ioannou, P.A. et al. A new bacteriophage P1-derived vector for the propagation of large human DNA fragments. Nature Genet. 6, 84–89 (1994).
Trask, B.J. et al. Characterization of somatic cell hybrids by bivariate flow karyotyping and fluorescence in situ hybridization. Somat. Cell Mol. Genet. 17, 117–136 (1991).
Town, M. et al. A novel gene encoding an integral membrane protein is mutated in nephropathic cystinosis. Nature Genet. 18, 319–324 (1998).
Li, B.L. et al. Human acyl-CoA:cholesterol acyltransferase-1 (ACAT-1) gene organization and evidence that the 4.3-kilobase ACAT-1 mRNA is produced from two different chromosomes. J. Biol. Chem. 274, 11060– 11071 (1999).
Brandenberger, A.W., Tee, M.K., Lee, J.Y., Chao, V. & Jaffe, R.B. Tissue distribution of estrogen receptors α (ER-α) and β (ER-β) mRNA in the midgestational human fetus. J. Clin. Endocrinol. Metab. 82, 3509– 3512 (1997).
Altschul, S.F., Gish, W., Miller, W., Myers, E.W. & Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 215, 403–410 ( 1990).
Thompson, J.D., Higgins, D.G. & Gibson, T.J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22, 4673–4680 (1994).
Fuchshuber, A. et al. Presymptomatic diagnosis of familial steroid-resistant nephrotic syndrome. Lancet 347, 1050– 1051 (1996).
Sibony, M., Commo, F., Callard, P. & Gasc, J.M. Enhancement of mRNA in situ hybridization signal by microwave heating. Lab. Invest. 73, 586–591 (1995).
Kalatzis, V., Sahly, I., El-Amraoui, A. & Petit, C. Eya1 expression in the developing ear and kidney: towards the understanding of the pathogenesis of branchio-oto-renal (BOR) syndrome. Dev. Dyn. 213, 486–499 ( 1998).
Heidet, L. et al. Diffuse leiomyomatosis associated with X-linked Alport syndrome: extracellular matrix study using immunohistochemistry and in situ hybridization. Lab. Invest. 76, 233–243 (1997).
Antonarakis, S.E. Recommendations for a nomenclature system for human gene mutations. Nomenclature Working Group. Hum. Mutat. 11, 1– 3 (1998).
Kaplan, J.M. et al. Mutations in ACTN4, encoding a-actinin 4, cause familial focal segmental glomerulosclerosis. Nature Genet. 64, 251–256 (2000).
Acknowledgements
We thank the patients and their families for participation; E. Al-Sabban, L. Alsford, J.L. André, F. Bouissou, S. Caliskan, E. Kuwertz-Bröcking, B. Lange, J. Nauta and W. Proesmans for referring patients; V. Kalatzis and L. Heidet for critical reading of the manuscript; V. Chauvet for help with in situ hybridization; and Y. Deris for assistance with figure preparation. This study was supported in part by the Association pour l'Utilisation du Rein Artificiel and the Fondation pour la Recherche Médicale. N.B. was supported by grants from the Association Française contre les Myopathies and, subsequently, the Programme Hospitalier de Recherche Clinique.
Author information
Authors and Affiliations
Corresponding author
Additional information
Note added in proof:
During the publishing process, J. Kaplan et al. have shown that mutations in ACTN4, mapped to 19q13 and encoding a-actinin-4, an actin-filament cross linking protein, cause autosomal FSGS (ref. 40).
Supplementary information
Rights and permissions
About this article
Cite this article
Boute, N., Gribouval, O., Roselli, S. et al. NPHS2, encoding the glomerular protein podocin, is mutated in autosomal recessive steroid-resistant nephrotic syndrome. Nat Genet 24, 349–354 (2000). https://doi.org/10.1038/74166
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/74166
This article is cited by
-
Copy number variation analysis in 138 families with steroid-resistant nephrotic syndrome identifies causal homozygous deletions in PLCE1 and NPHS2 in two families
Pediatric Nephrology (2024)
-
In vivo characterization of a podocyte-expressed short podocin isoform
BMC Nephrology (2023)
-
Anti-VEGFR2 F(ab′)2 drug conjugate promotes renal accumulation and glomerular repair in diabetic nephropathy
Nature Communications (2023)
-
Hidden genetics behind glomerular scars: an opportunity to understand the heterogeneity of focal segmental glomerulosclerosis?
Pediatric Nephrology (2023)
-
The dominant findings of a recessive man: from Mendel’s kid pea to kidney
Pediatric Nephrology (2023)