Abstract
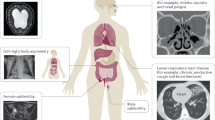
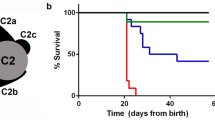
Primary ciliary dyskinesia (PCD) is a genetically heterogeneous autosomal recessive disorder characterized by recurrent infections of the respiratory tract associated with the abnormal function of motile cilia. Approximately half of individuals with PCD also have alterations in the left-right organization of their internal organ positioning, including situs inversus and situs ambiguous (Kartagener's syndrome). Here, we identify an uncharacterized coiled-coil domain containing a protein, CCDC40, essential for correct left-right patterning in mouse, zebrafish and human. In mouse and zebrafish, Ccdc40 is expressed in tissues that contain motile cilia, and mutations in Ccdc40 result in cilia with reduced ranges of motility. We further show that CCDC40 mutations in humans result in a variant of PCD characterized by misplacement of the central pair of microtubules and defective assembly of inner dynein arms and dynein regulatory complexes. CCDC40 localizes to motile cilia and the apical cytoplasm and is required for axonemal recruitment of CCDC39, disruption of which underlies a similar variant of PCD.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Fliegauf, M., Benzing, T. & Omran, H. When cilia go bad: cilia defects and ciliopathies. Nat. Rev. Mol. Cell Biol. 8, 880–893 (2007).
Barbato, A. et al. Primary ciliary dyskinesia: a consensus statement on diagnostic and treatment approaches in children. Eur. Respir. J. 34, 1264–1276 (2009).
Duquesnoy, P. et al. Loss-of-function mutations in the human ortholog of Chlamydomonas reinhardtii ODA7 disrupt dynein arm assembly and cause primary ciliary dyskinesia. Am. J. Hum. Genet. 85, 890–896 (2009).
Loges, N.T. et al. Deletions and point mutations of LRRC50 cause primary ciliary dyskinesia due to dynein arm defects. Am. J. Hum. Genet. 85, 883–889 (2009).
Omran, H. et al. Ktu/PF13 is required for cytoplasmic pre-assembly of axonemal dyneins. Nature 456, 611–616 (2008).
van Rooijen, E. et al. LRRC50, a conserved ciliary protein implicated in polycystic kidney disease. J. Am. Soc. Nephrol. 19, 1128–1138 (2008).
Zohn, I.E., Anderson, K.V. & Niswander, L. Using genomewide mutagenesis screens to identify the genes required for neural tube closure in the mouse. Birth Defects Res. A Clin. Mol. Teratol. 73, 583–590 (2005).
García-García, M.J. et al. Analysis of mouse embryonic patterning and morphogenesis by forward genetics. Proc. Natl. Acad. Sci. USA 102, 5913–5919 (2005).
Ibañez-Tallon, I. et al. Dysfunction of axonemal dynein heavy chain Mdnah5 inhibits ependymal flow and reveals a novel mechanism for hydrocephalus formation. Hum. Mol. Genet. 13, 2133–2141 (2004).
Burkhard, P., Stetefeld, J. & Strelkov, S.V. Coiled coils: a highly versatile protein folding motif. Trends Cell Biol. 11, 82–88 (2001).
Sullivan-Brown, J. et al. Zebrafish mutations affecting cilia motility share similar cystic phenotypes and suggest a mechanism of cyst formation that differs from pkd2 morphants. Dev. Biol. 314, 261–275 (2008).
Zhao, C. & Malicki, J. Genetic defects of pronephric cilia in zebrafish. Mech. Dev. 124, 605–616 (2007).
Merveille, A.-C. et al. CCDC39 is required for assembly of inner dynein arms and the dynein regulatory complex and for normal ciliary motility in humans and dogs. Nat. Genet. advance online publication, doi:10.1038/ng.726 (5 December 2010).
Colantonio, J.R. et al. Expanding the role of the dynein regulatory complex to non-axonemal functions: association of GAS11 with the Golgi apparatus. Traffic 7, 538–548 (2006).
Huang, B., Ramanis, Z. & Luck, D.J. Suppressor mutations in Chlamydomonas reveal a regulatory mechanism for flagellar function. Cell 28, 115–124 (1982).
Piperno, G., Mead, K., LeDizet, M. & Moscatelli, A. Mutations in the “dynein regulatory complex” alter the ATP-insensitive binding sites for inner arm dyneins in Chlamydomonas axonemes. J. Cell Biol. 125, 1109–1117 (1994).
Piperno, G., Mead, K. & Shestak, W. The inner dynein arms I2 interact with a “dynein regulatory complex” in Chlamydomonas flagella. J. Cell Biol. 118, 1455–1463 (1992).
Kamiya, R. Functional diversity of axonemal dyneins as studied in Chlamydomonas mutants. Int. Rev. Cytol. 219, 115–155 (2002).
Zariwala, M.A., Knowles, M.R. & Omran, H. Genetic defects in ciliary structure and function. Annu. Rev. Physiol. 69, 423–450 (2007).
Fliegauf, M. et al. Mislocalization of DNAH5 and DNAH9 in respiratory cells from patients with primary ciliary dyskinesia. Am. J. Respir. Crit. Care Med. 171, 1343–1349 (2005).
Sisson, J.H., Stoner, J.A., Ammons, B.A. & Wyatt, T.A. All-digital image capture and whole-field analysis of ciliary beat frequency. J. Microsc. 211, 103–111 (2003).
Holmes, G. & Niswander, L. Expression of slit-2 and slit-3 during chick development. Dev. Dyn. 222, 301–307 (2001).
Liu, A., Joyner, A.L. & Turnbull, D.H. Alteration of limb and brain patterning in early mouse embryos by ultrasound-guided injection of Shh-expressing cells. Mech. Dev. 75, 107–115 (1998).
Timmer, J.R., Wang, C. & Niswander, L. BMP signaling patterns the dorsal and intermediate neural tube via regulation of homeobox and helix-loop-helix transcription factors. Development 129, 2459–2472 (2002).
Schottenfeld, J., Sullivan-Brown, J. & Burdine, R.D. Zebrafish curly up encodes a Pkd2 ortholog that restricts left-side-specific expression of southpaw. Development 134, 1605–1615 (2007).
Thisse, C. & Thisse, B. High-resolution in situ hybridization to whole-mount zebrafish embryos. Nat. Protoc. 3, 59–69 (2008).
Jaffe, K.M., Thiberge, S.Y., Bisher, M.E. & Burdine, R.D. Imaging cilia in zebrafish. Methods Cell Biol. 97, 415–435 (2010).
Acknowledgements
We thank the German subject support group 'Kartagener Syndrom und Primaere Ciliaere Dyskinesie e.V.', S. Glaser for the initial genomic mapping of lok, J. Liu for help with imaging Ccdc40 protein expression in the mouse node, L. Bulwith, A. Heer, C. Kopp, D. Nergenau and K. Sutter for excellent technical assistance, and D. Bosco for zebrafish facility maintenance. We also thank M. Griese (Munich), E.v. Mutius (Munich), T. Nuesslein (Koblenz), N. Schwerk (Hannover), S. Reithmayr (Vienna), H. Seithe (Nuernberg) and M. Stern (Tuebingen) for supporting the study. The lnks mutant mouse line was established as part of the Sloan-Kettering Institute Mouse Project (R37-HD035455). This work was supported by the Basil O'Connor Award from the March of Dimes, the Young Investigator Award from the Spina Bifida Association and R01-HD058629 to I.E.Z.; the German Human Genome Project DHGP grant 01 KW9919 to R.G.; the Howard Hughes Medical Institute to L.N.; 'Deutsche Forschungsgemeinschaft' DFG Om 6/4, GRK1104 and the SFB592 to H.O.; and NICHD-R01HD048584 to R.D.B.
Author information
Authors and Affiliations
Contributions
Studies in mice were conducted by I.E.Z., A.P., A.B.-H., H. Omran, K.V.A. and L.N. Studies in zebrafish were conducted by N.O., K.B.L., J.S.-B., J.M., R.G. and R.D.B. Studies with human samples were conducted by A.B.-H., N.T.L., H. Olbrich, K.H., M.F., J.H., R.R., K.G.N., J.K.M., G.B. and H. Omran. The manuscript was prepared by A.B.-H., I.E.Z., L.N., H. Omran and R.D.B.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8 and Supplementary Table 1 (PDF 9961 kb)
Supplementary Video 1
Cilia motility in control cells from a nasal brush biopsy (AVI 3432 kb)
Supplementary Video 2
Defective cilia motility in patient OP76II1 (AVI 2086 kb)
Supplementary Video 3
Defective cilia motility in patient OP82II1 (AVI 5697 kb)
Supplementary Video 4
Defective cilia motility in patient OP87II2 (AVI 3471 kb)
Rights and permissions
About this article
Cite this article
Becker-Heck, A., Zohn, I., Okabe, N. et al. The coiled-coil domain containing protein CCDC40 is essential for motile cilia function and left-right axis formation. Nat Genet 43, 79–84 (2011). https://doi.org/10.1038/ng.727
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng.727
This article is cited by
-
Characterization of a DRC1 null variant associated with primary ciliary dyskinesia and female infertility
Journal of Assisted Reproduction and Genetics (2023)
-
Dynein axonemal heavy chain 10 deficiency causes primary ciliary dyskinesia in humans and mice
Frontiers of Medicine (2023)
-
Lack of CFAP54 causes primary ciliary dyskinesia in a mouse model and human patients
Frontiers of Medicine (2023)
-
The dynamics of protein localisation to restricted zones within Drosophila mechanosensory cilia
Scientific Reports (2022)
-
A change of heart: new roles for cilia in cardiac development and disease
Nature Reviews Cardiology (2022)