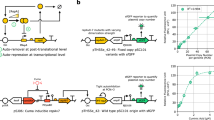
Abstract
A defining goal of synthetic biology is to engineer cells to coordinate tasks that often require precise temporal modulation of gene expression. Although a variety of relatively small gene circuits have been constructed and characterized, their logical combination into larger networks remains a central challenge. This is due primarily to the lack of compatible and orthogonal elements for predictable dynamic control of gene expression. As an alternative approach to promoter-level regulation, we explored the use of DNA copy number as a circuit control element. We engineered colony-wide DNA cycling in Escherichia coli in the form of plasmid copy number oscillations via a modular design that can be readily adapted for use with other gene circuitry. Copy number modulation is a generalizable principle that adds a layer of control to synthetic gene circuits, allowing dynamic regulation of circuit elements without requiring specially engineered promoters.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Slager, J., Kjos, M., Attaiech, L. & Veening, J.-W. Antibiotic-induced replication stress triggers bacterial competence by increasing gene dosage near the origin. Cell 157, 395–406 (2014).
Narula, J. et al. Chromosomal arrangement of phosphorelay genes couples sporulation and DNA replication. Cell 162, 328–337 (2015).
Marguet, P., Tanouchi, Y., Spitz, E., Smith, C. & You, L. Oscillations by minimal bacterial suicide circuits reveal hidden facets of host-circuit physiology. PLoS One 5, e11909 (2010).
Danino, T., Mondragón-Palomino, O., Tsimring, L. & Hasty, J. A synchronized quorum of genetic clocks. Nature 463, 326–330 (2010).
Stevens, A.M. & Greenberg, E.P. Quorum sensing in Vibrio fischeri: essential elements for activation of the luminescence genes. J. Bacteriol. 179, 557–562 (1997).
Gottesman, S., Roche, E., Zhou, Y. & Sauer, R.T. The ClpXP and ClpAP proteases degrade proteins with carboxy-terminal peptide tails added by the SsrA-tagging system. Genes Dev. 12, 1338–1347 (1998).
Wróbel, B. & We¸grzyn, G. Replication regulation of ColE1-like plasmids in amino wacid-starved Escherichia coli. Plasmid 39, 48–62 (1998).
Tomizawa, J. Control of ColE1 plasmid replication: binding of RNA I to RNA II and inhibition of primer formation. Cell 47, 89–97 (1986).
Selzer, G., Som, T., Itoh, T. & Tomizawa, J. The origin of replication of plasmid p15A and comparative studies on the nucleotide sequences around the origin of related plasmids. Cell 32, 119–129 (1983).
Panayotatos, N. DNA replication regulated by the priming promoter. Nucleic Acids Res. 12, 2641–2648 (1984).
Stricker, J. et al. A fast, robust and tunable synthetic gene oscillator. Nature 456, 516–519 (2008).
Mather, W., Bennett, M.R., Hasty, J. & Tsimring, L.S. Delay-induced degrade-and-fire oscillations in small genetic circuits. Phys. Rev. Lett. 102, 068105 (2009).
Cookson, N.A. et al. Queueing up for enzymatic processing: correlated signaling through coupled degradation. Mol. Syst. Biol. 7, 561 (2011).
Elowitz, M.B. & Leibler, S. A synthetic oscillatory network of transcriptional regulators. Nature 403, 335–338 (2000).
Gardner, T.S., Cantor, C.R. & Collins, J.J. Construction of a genetic toggle switch in Escherichia coli. Nature 403, 339–342 (2000).
Roquet, N. & Lu, T.K. Digital and analog gene circuits for biotechnology. Biotechnol. J. 9, 597–608 (2014).
Brophy, J.A. & Voigt, C.A. Principles of genetic circuit design. Nat. Methods 11, 508–520 (2014).
Rubens, J.R., Selvaggio, G. & Lu, T.K. Synthetic mixed-signal computation in living cells. Nat. Commun. 7, 11658 (2016).
You, L., Cox, R.S. III, Weiss, R. & Arnold, F.H. Programmed population control by cell-cell communication and regulated killing. Nature 428, 868–871 (2004).
Prindle, A. et al. A sensing array of radically coupled genetic 'biopixels'. Nature 481, 39–44 (2011).
Basu, S., Gerchman, Y., Collins, C.H., Arnold, F.H. & Weiss, R. A synthetic multicellular system for programmed pattern formation. Nature 434, 1130–1134 (2005).
Moon, T.S., Lou, C., Tamsir, A., Stanton, B.C. & Voigt, C.A. Genetic programs constructed from layered logic gates in single cells. Nature 491, 249–253 (2012).
Purcell, O. & Lu, T.K. Synthetic analog and digital circuits for cellular computation and memory. Curr. Opin. Biotechnol. 29, 146–155 (2014).
Xie, Z., Wroblewska, L., Prochazka, L., Weiss, R. & Benenson, Y. Multi-input RNAi-based logic circuit for identification of specific cancer cells. Science 333, 1307–1311 (2011).
Kobayashi, H. et al. Programmable cells: interfacing natural and engineered gene networks. Proc. Natl. Acad. Sci. USA 101, 8414–8419 (2004).
Friedland, A.E. et al. Synthetic gene networks that count. Science 324, 1199–1202 (2009).
Tamsir, A., Tabor, J.J. & Voigt, C.A. Robust multicellular computing using genetically encoded NOR gates and chemical 'wires'. Nature 469, 212–215 (2011).
Tabor, J.J. et al. A synthetic genetic edge detection program. Cell 137, 1272–1281 (2009).
Bonnet, J., Yin, P., Ortiz, M.E., Subsoontorn, P. & Endy, D. Amplifying genetic logic gates. Science 340, 599–603 (2013).
Siuti, P., Yazbek, J. & Lu, T.K. Synthetic circuits integrating logic and memory in living cells. Nat. Biotechnol. 31, 448–452 (2013).
Bonnet, J., Subsoontorn, P. & Endy, D. Rewritable digital data storage in live cells via engineered control of recombination directionality. Proc. Natl. Acad. Sci. USA 109, 8884–8889 (2012).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012).
Škulj, M. et al. Improved determination of plasmid copy number using quantitative real-time PCR for monitoring fermentation processes. Microb. Cell Fact. 7, 6 (2008).
Hoops, S. et al. COPASI—a complex pathway simulator. Bioinformatics 22, 3067–3074 (2006).
Acknowledgements
We thank R. Johnson for assistance with microfluidic device design and fabrication, and P.J. Steiner and J. Humphries for helpful input and discussions. We are also grateful to the laboratory of B. Palsson for generously providing access to real-time PCR equipment. This work was supported by the NSF (award MCB-1616997 to J.H. and L.B.; award MCB-1330180 to W.M.).
Author information
Authors and Affiliations
Contributions
L.B. and J.H. contributed to the circuit design and development of the project. L.B. constructed the plasmids and strains, conducted the experiments, and analyzed results. W.M. and J.H. conducted the mathematical analysis and computational modeling. L.B. and W.M. prepared the figures, and L.B., W.M., and J.H. prepared the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Plasmid diagrams of primary strains used in this work.
Refer to Supplementary Table 1 for additional information and full list of strains. Diagrams are not to scale. (a) Strain used to demonstrate and quantify ColE1 copy number repression by I-SceI. A, B, and C refers to primer sets used for qPCR as shown in Supplementary Figure 2. (b) Plasmid copy number oscillator strain without RNAII overexpression. Note the transcriptional terminator downstream of SCEI preventing transcription from progressing into the p15A origin. (c) Strain used to visualize and quantify p15A copy number amplification by RNAII overproduction. The transcriptional terminator before the p15A origin has been replaced with a second copy of the luxI promoter, and the SCEI gene with a gfp gene. (d) Plasmid copy number oscillator strain modified to include RNAII overproduction under control of the luxI promoter as in LABS3.
Supplementary Figure 2 qPCR analysis of ColE1 copy number repression.
qPCR analysis of ColE1 copy number repression after I-SceI induction with 0.2% w/v arabinose for 3 hours, using multiple primer sets annealing at different locations around the plasmid. Results demonstrate that after being cut by I-SceI, the majority of linear plasmids are quickly degraded. Primer set A is the same that used to quantify copy number in Figure 1, a. Mean and s.e.m. are displayed. Statistical significance was calculated by independent 2-sample t test with d.f. = 18, yielding P = 6 × 10-12 for primer set A, P = 9 × 10-7 for primer set B, and P = 2 × 10-7 for primer set C. See Methods for detailed description of experimental design, Supplementary Figure 1 for detailed plasmid diagrams, and Supplementary Table 2 for primer sequences.
Supplementary Figure 3 Schematic of microfluidic device 1.
Each flow channel feeds growth medium to a single cell chamber (displayed in blue), preventing AHL diffusion between individual chambers. Flat rectangular chambers (x, y, z = 100 μm, 85 μm, 1.6 μm) allow visualization of single E. coli cells. Flow channels are 30 μm high.
Supplementary Figure 4 qPCR analysis of p15A copy number amplification by RNAII overexpression.
Copy number amplification after 90 minutes of induction with 450 nM AHL, where Pluxl promoter induction leads to RNAII overexpression from the p15A origin. Mean and s.e.m. are displayed. Statistical significance was calculated by independent 2-sample t test with d.f. = 18, yielding P = 3 × 10-8 for plasmid copy number and P = 3 × 10-4 for RFP fluorescence. See Methods for detailed description of experimental design, Supplementary Figure 1 for detailed plasmid diagrams, and Supplementary Table 2 for primer sequences.
Supplementary Figure 5 Schematic of microfluidic device 2.
Each flow channel feeds growth medium to a single cell chamber (displayed in blue), preventing AHL diffusion between individual chambers. Larger and taller chambers (x, y, z = 15 μm, 100 μm, 50 μm) allow exploration of circuit dynamics in an alternate geometry. Flow channels are the same height as chambers at 50 μm.
Supplementary Figure 6 PCA analysis of sensitive parameters.
Robustness analysis was done to identify which parameters most sensitively control oscillations (see Supplementary Note for details). For (a) model 1 and (b) model 2, we formed non-oscillatory parameter sets from the data used to generate Figure 3, e, and we determined the principal components with the lowest coefficient of variation (a proxy for sensitivity in this set). We examined representative non-oscillatory sets using η = 0.3 and η = 0.1 when studying model 1 and model 2, respectively, containing non-oscillatory parameter set sizes of 5,869 and 6,468, respectively. The principal component with the least coefficient of variation was found using standard techniques, the absolute magnitude for each parameter value was taken, and the sum of principal components was normalized to 1. For each model, this process was repeated using 1,000 ensembles containing a random 20% of the full non-oscillatory set. We applied bootstrapping to determine the mean (blue bars) and standard deviation (red lines) of the principal component with the least coefficient of variation. Overall, the enzymatic velocity μ was consistently a sensitive parameter.
Supplementary Figure 7 The fraction of oscillatory models with a scan of two oscillator parameters.
(a) The cutting rate constant, kc, for I-SceI was varied from 0 to 10-times its best fit value. All other parameters were varied using a uniform distribution ranging ± 5% (similarly as done for Figure 3, e for η = 0.10, but using 100 ensembles of size 100 each to estimate error). The dashed line indicates the best fit value. (b) We similarly investigated robustness for the cooperativity coefficient n, which was scanned from 1 to 8. The observation that model 2 for both (a) and (b) has fewer nearby parameter sets that oscillate in the vicinity of the best fit is consistent with the picture that model 2 is tuned closer to a bifurcation point.
Supplementary Figure 8 Models addressing experimental robustness observations.
To address the observations in Figure 3, where only the circuit with RNAII overexpression feedback oscillated, we examined our model for parameters modified to reflect a change in microfluidic chamber geometry, e.g. leading to slower AHL buildup in the chamber. In particular, we effectively modified the level of LuxI needed to activate the circuit by increasing the parameter value for A0 by 2-fold, and we modified the delay for positive feedback by increasing the parameter value for ktauA by 6-fold. (a) Model 1 did not exhibit oscillations after a short transient, while model 2 exhibited sustained oscillations, as is consistent with experiment. (b) Plasmid copy numbers maintained reasonable values, suggesting plasmid extinction should not be a concern. (c) These solutions were reasonably robust with respect to general parameter variation. Robustness analysis was performed as in Figure 3, e.
Supplementary Figure 9 Transcription factor titration by plasmid copy number amplification.
p15A copy number amplification counteracts positive feedback from the activator plasmid even without cutting by I-SceI. The activator plasmid used here lacks an I-SceI cut site. The repressor plasmid has been modified to allow inducible plasmid amplification with IPTG, driven by the lac-repressible PLlacO1 promoter. Both luxI promoters are left intact, each containing a single LuxR binding site, however the transcriptional terminator downstream of the luxI promoters prevents transcription from progressing into the p15A origin. RFP reports on p15A copy number and GFP on lux activation state. A small amount of AHL (5 nM) is introduced at t = 1 hour to start lux positive feedback from the activator plasmid, causing a rise in GFP signal. At t = 2 hours, 100 μM IPTG is added in addition, causing amplification of the p15A copy number as seen by the rising RFP signal. IPTG is removed again at t = 6 hours, which allows p15A copy number to slowly drop back to natural levels by dilution due to cell division, at which point positive feedback from the activator plasmid resumes.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–9, Supplementary Tables 1–2, and Supplementary Note 1. (PDF 1583 kb)
Oscillator circuit 1
Synthetic quorum oscillator circuit without RNAII overexpression feedback expressed in E. coli growing under constant conditions in a microfluidic chamber. (MP4 24315 kb)
Oscillator circuit 2
Synthetic quorum oscillator circuit modified to include RNAII overexpression feedback on the repressor plasmid, expressed in E. coli growing under constant conditions in a microfluidic chamber. (MP4 28365 kb)
Oscillator circuit 2 in large chambers
Oscillator circuit with RNAII overexpression feedback, in E. coli growing in a larger microfluidic chamber with rich medium. (MP4 12913 kb)
Comparison of oscillator circuit robustness
Comparison of circuit 1 without RNAII overexpression (top) and circuit 2 with RNAII overexpression (bottom) in E. coli growing in the larger microfluidic chambers with rich medium. To allow for direct comparison, we acquired images with the same microscope and exposure settings for both circuits. (MP4 936 kb)
Rights and permissions
About this article
Cite this article
Baumgart, L., Mather, W. & Hasty, J. Synchronized DNA cycling across a bacterial population. Nat Genet 49, 1282–1285 (2017). https://doi.org/10.1038/ng.3915
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng.3915
This article is cited by
-
Addressable and adaptable intercellular communication via DNA messaging
Nature Communications (2023)
-
Customizing cellular signal processing by synthetic multi-level regulatory circuits
Nature Communications (2023)
-
Inducible plasmid copy number control for synthetic biology in commonly used E. coli strains
Nature Communications (2022)
-
Majority sensing in synthetic microbial consortia
Nature Communications (2020)
-
Quorum sensing for population-level control of bacteria and potential therapeutic applications
Cellular and Molecular Life Sciences (2020)