Abstract
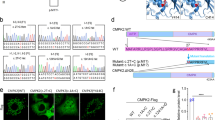
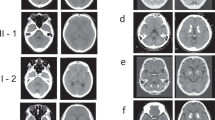
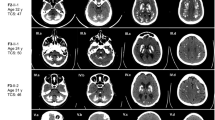
Calcifications in the basal ganglia are a common incidental finding and are sometimes inherited as an autosomal dominant trait (idiopathic basal ganglia calcification (IBGC)). Recently, mutations in the PDGFRB gene coding for the platelet-derived growth factor receptor β (PDGF-Rβ) were linked to IBGC. Here we identify six families of different ancestry with nonsense and missense mutations in the gene encoding PDGF-B, the main ligand for PDGF-Rβ. We also show that mice carrying hypomorphic Pdgfb alleles develop brain calcifications that show age-related expansion. The occurrence of these calcium depositions depends on the loss of endothelial PDGF-B and correlates with the degree of pericyte and blood-brain barrier deficiency. Thus, our data present a clear link between Pdgfb mutations and brain calcifications in mice, as well as between PDGFB mutations and IBGC in humans.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Accession codes
References
Förstl, H., Krumm, B., Eden, S. & Kohlmeyer, K. Neurological disorders in 166 patients with basal ganglia calcification: a statistical evaluation. J. Neurol. 239, 36–38 (1992).
Yamada, M. et al. High frequency of calcification in basal ganglia on brain computed tomography images in Japanese older adults. Geriatr. Gerontol. Int. 13, 706–710 (2013).
Oliveira, J.R.M.D. Managing Idiopathic Basal Ganglia Calcification (“Fahr's Disease”) (Nova Science Publishers, New York, 2011).
Wang, C. et al. Mutations in SLC20A2 link familial idiopathic basal ganglia calcification with phosphate homeostasis. Nat. Genet. 44, 254–256 (2012).
Hsu, S.C. et al. Mutations in SLC20A2 are a major cause of familial idiopathic basal ganglia calcification. Neurogenetics 14, 11–22 (2013).
Nicolas, G. et al. Mutation of the PDGFRB gene as a cause of idiopathic basal ganglia calcification. Neurology 80, 181–187 (2013).
Lemos, R.R., Oliveira, M.F. & Oliveira, J.R. Reporting a new mutation at the SLC20A2 gene in familial idiopathic basal ganglia calcification. Eur. J. Neurol. 20, e43–e44 (2013).
Zhang, Y., Guo, X. & Wu, A. Association between a novel mutation in SLC20A2 and familial idiopathic basal ganglia calcification. PLoS ONE 8, e57060 (2013).
Andrae, J., Gallini, R. & Betsholtz, C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev. 22, 1276–1312 (2008).
Levéen, P. et al. Mice deficient for PDGF B show renal, cardiovascular, and hematological abnormalities. Genes Dev. 8, 1875–1887 (1994).
Soriano, P. Abnormal kidney development and hematological disorders in PDGF β–receptor mutant mice. Genes Dev. 8, 1888–1896 (1994).
Lindahl, P., Johansson, B.R., Levéen, P. & Betsholtz, C. Pericyte loss and microaneurysm formation in PDGF-B–deficient mice. Science 277, 242–245 (1997).
Hellström, M., Kalén, M., Lindahl, P., Abramsson, A. & Betsholtz, C. Role of PDGF-B and PDGFR-β in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development 126, 3047–3055 (1999).
Lindblom, P. et al. Endothelial PDGF-B retention is required for proper investment of pericytes in the microvessel wall. Genes Dev. 17, 1835–1840 (2003).
Bjarnegård, M. et al. Endothelium-specific ablation of PDGFB leads to pericyte loss and glomerular, cardiac and placental abnormalities. Development 131, 1847–1857 (2004).
Enge, M. et al. Endothelium-specific platelet-derived growth factor-B ablation mimics diabetic retinopathy. EMBO J. 21, 4307–4316 (2002).
Tallquist, M.D., French, W.J. & Soriano, P. Additive effects of PDGF receptor β signaling pathways in vascular smooth muscle cell development. PLoS Biol. 1, E52 (2003).
Daneman, R., Zhou, L., Kebede, A.A. & Barres, B.A. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature 468, 562–566 (2010).
Armulik, A. et al. Pericytes regulate the blood-brain barrier. Nature 468, 557–561 (2010).
Kostić, V.S. et al. Exclusion of linkage to chromosomes 14q, 2q37 and 8p21.1-q11.23 in a Serbian family with idiopathic basal ganglia calcification. J. Neurol. 258, 1637–1642 (2011).
Heldin, C.H. & Westermark, B. Mechanism of action and in vivo role of platelet-derived growth factor. Physiol. Rev. 79, 1283–1316 (1999).
Wise, R.J., Orkin, S.H. & Collins, T. Aberrant expression of platelet-derived growth factor A–chain cDNAs due to cryptic splicing of RNA transcripts in COS-1 cells. Nucleic Acids Res. 17, 6591–6601 (1989).
Abramsson, A. et al. Defective N-sulfation of heparan sulfate proteoglycans limits PDGF-BB binding and pericyte recruitment in vascular development. Genes Dev. 21, 316–331 (2007).
Miklossy, J. et al. Severe vascular disturbance in a case of familial brain calcinosis. Acta Neuropathol. 109, 643–653 (2005).
Saitou, M. et al. Complex phenotype of mice lacking occludin, a component of tight junction strands. Mol. Biol. Cell 11, 4131–4142 (2000).
O'Driscoll, M.C. et al. Recessive mutations in the gene encoding the tight junction protein occludin cause band-like calcification with simplified gyration and polymicrogyria. Am. J. Hum. Genet. 87, 354–364 (2010).
Kalueff, A. et al. Thalamic calcification in vitamin D receptor knockout mice. Neuroreport 17, 717–721 (2006).
Chakrabarty, P. et al. Interferon-γ induces progressive nigrostriatal degeneration and basal ganglia calcification. Nat. Neurosci. 14, 694–696 (2011).
Mann, D.M. Calcification of the basal ganglia in Down's syndrome and Alzheimer's disease. Acta Neuropathol. 76, 595–598 (1988).
Ramonet, D. et al. Similar calcification process in acute and chronic human brain pathologies. J. Neurosci. Res. 83, 147–156 (2006).
Vermersch, P., Leys, D., Pruvo, J.P., Clarisse, J. & Petit, H. Parkinson's disease and basal ganglia calcifications: prevalence and clinico-radiological correlations. Clin. Neurol. Neurosurg. 94, 213–217 (1992).
Nagaratnam, N. & Plew, J.D. Extensive intracranial calcification secondary to hypoxia, presenting with dyspraxic gait. Australas. Radiol. 42, 232–233 (1998).
Liu, X., Jian, X. & Boerwinkle, E. dbNSFP: a lightweight database of human nonsynonymous SNPs and their functional predictions. Hum. Mutat. 32, 894–899 (2011).
Davydov, E.V. et al. Identifying a high fraction of the human genome to be under selective constraint using GERP++. PLoS Comput. Biol. 6, e1001025 (2010).
Kisanuki, Y.Y. et al. Tie2-Cre transgenic mice: a new model for endothelial cell–lineage analysis in vivo. Dev. Biol. 230, 230–242 (2001).
Grünecker, B. et al. Fractionated manganese injections: effects on MRI contrast enhancement and physiological measures in C57BL/6 mice. NMR Biomed. 23, 913–921 (2010).
Acknowledgements
We wish to thank the participating family members for their valuable collaboration. We are thankful to B. Sobrino and J. Amigo for their help with exome sequencing, P. Cacheiro and I. König for help with data analysis and M. Delic and M. König for technical assistance. A. Keller holds a Marie Heim-Vögtlin fellowship from the Swiss National Science Foundation. A.W. is supported by the Fritz Thyssen Foundation, a Jake's Ride for Dystonia research grant through the Bachmann-Strauss Dystonia & Parkinson Foundation, a Habilitation Fellowship for Women Researchers (E26-2011) and by the Medical Genetics Priority Program from the University of Lübeck, Germany. M.J.S., M.G.-M., A.O.-U. and A.C. are supported by a research grant from the Xunta de Galicia, Consellería de Innovación (10PXIB9101280PR) and by European Regional Development (FEDER) funds. M.J.S. is the recipient of a research contract from the Institute of Health Carlos III. J.R.M.O., R.R.L. and J.E.G.d.C. are supported by grants from the John Simon Guggenheim Memorial Foundation, Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Fundacão de Amparo à Ciênta e Tecnologia do Estado de Pernamnbuco (FACEPE) and Coordenacão de Aperfeicoamento de Pessoal de Nível Superior (CAPES). G.N., D.H. and D.C. are supported by funding of Centre National de Référence pour les Malades Alzheimer Jeunes (CNR-MAJ) by the French Ministry of Health and by the Rouen University Hospital. M.C.W. (Swiss National Science Foundation grant 310030_144075/1SNR) and the micro-CT unit (R'Equipe grant 3106030_139258/1) were supported by grants from the Swiss National Science Foundation. K.L. is supported by two research grants from the German Research Foundation (DFG) and by the Medical Genetics Priority Program of the University of Lübeck, Germany. V.D., I.P., M.J., I. Novaković and V.S.K. are supported by a research grant from the Serbian Ministry of Education and Science (project grant 175090). M.Z. is supported by FAPESP/389 CEPID (State of São Paulo Research Foundation and CNPq (Instituto Nacional de Ciência e Tecnologia de Células Tronco em Doenças Genéticas Humanas)). K.Z. is supported by a research grant from the University of Lübeck (E30/2011). A.A. is the recipient of an Advanced Grant of the European Research Council and is supported by grants from the European Union (PRIORITY and LUPAS), the Swiss National Science Foundation, the Foundation Alliance BioSecure, the Clinical Research Focus Program of the University of Zürich and the Novartis Research Foundation. D.H.G. was supported by the US National Institutes of Health/National Institute of Neurological Disorders and Stroke (NIH/NINDS; R01 NS040752). C.B. is the recipient of an Advanced Grant of the European Research Council and is supported by grants from Uppsala University, the Knut and Alice Wallenberg Foundation, the Torsten and Ragnar Söderberg Foundation, the IngaBritt and Arne Lundberg Foundation, the Swedish Research Council, the Swedish Cancer Society and the Cardiovascular Program at Karolinska Institutet. C.K. is supported by a career development award from the Hermann and Lilly Schilling Foundation and by the Medical Genetics Priority Program of the University of Lübeck, Germany.
Author information
Authors and Affiliations
Contributions
J.R.M.O., C.B., C.K. and V.S.K. initiated the project, which was subsequently developed and led jointly by A. Keller, A.W., M.J.S., C.B., C.K. and J.R.M.O. A. Keller, A.W., M.J.S., K.L., K.Z., I. Navas, C.B., C.K. and J.R.M.O. conceived the experiments. A. Keller, E.J.R., M.H., R.R., I.A., M.A.M., E.R., M.C.W., A.B. and A. Kaech performed the mouse experiments, which were financially supported by C.B. and A.A. A.W., M.J.S., M.G.-M., A.D., R.L.S., R.R.L., A.O.-U., G.N., J.E.G.d.C., K.L., V.D., A.C., I.P., J.M.M., M.Z., K.Z., J.K., E.S., J.M.P., I. Navas, M. Preuss, C.D., M.J., M. Paucar, P.S., K.S., H.R.K.K., I. Novaković, I.L.B., G.D., D.H., V.S.K., D.C., D.H.G., G.C., C.K. and J.R.M.O. recruited and examined patients, collected and analyzed human DNA and/or interpreted genetic data. C.B., A. Keller, A.W., M.J.S., C.K. and J.R.M.O. wrote the manuscript with critical input from A.D., K.L. and K.Z.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–3, Supplementary Tables 1–5 and Supplementary Note (PDF 4300 kb)
Rights and permissions
About this article
Cite this article
Keller, A., Westenberger, A., Sobrido, M. et al. Mutations in the gene encoding PDGF-B cause brain calcifications in humans and mice. Nat Genet 45, 1077–1082 (2013). https://doi.org/10.1038/ng.2723
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng.2723
This article is cited by
-
JAM2 variants can be more common in primary familial brain calcification (PFBC) cases than those appear; may be due to a founder mutation
Neurological Sciences (2024)
-
One Train May Hide Another: Two Cases of Co-Occurring Primary Familial Brain Calcification and Alzheimer’s Disease
Journal of Molecular Neuroscience (2024)
-
Aneurysmal subarachnoid hemorrhage with PFBC and beta thalassemia: a case report
BMC Neurology (2023)
-
The CLDN5 gene at the blood-brain barrier in health and disease
Fluids and Barriers of the CNS (2023)
-
The clinical and genetic spectrum of primary familial brain calcification
Journal of Neurology (2023)