Abstract
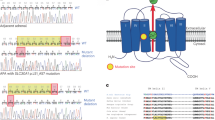
Adrenal aldosterone-producing adenomas (APAs) constitutively produce the salt-retaining hormone aldosterone and are a common cause of severe hypertension. Recurrent mutations in the potassium channel gene KCNJ5 that result in cell depolarization and Ca2+ influx cause ∼40% of these tumors1. We identified 5 somatic mutations (4 altering Gly403 and 1 altering Ile770) in CACNA1D, encoding a voltage-gated calcium channel, among 43 APAs without mutated KCNJ5. The altered residues lie in the S6 segments that line the channel pore. Both alterations result in channel activation at less depolarized potentials; Gly403 alterations also impair channel inactivation. These effects are inferred to cause increased Ca2+ influx, which is a sufficient stimulus for aldosterone production and cell proliferation in adrenal glomerulosa2. We also identified de novo germline mutations at identical positions in two children with a previously undescribed syndrome featuring primary aldosteronism and neuromuscular abnormalities. These findings implicate gain-of-function Ca2+ channel mutations in APAs and primary aldosteronism.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
Primary accessions
GenBank/EMBL/DDBJ
Referenced accessions
NCBI Reference Sequence
Swiss-Prot
References
Choi, M. et al. K+ channel mutations in adrenal aldosterone-producing adenomas and hereditary hypertension. Science 331, 768–772 (2011).
Spät, A. & Hunyady, L. Control of aldosterone secretion: a model for convergence in cellular signaling pathways. Physiol. Rev. 84, 489–539 (2004).
Rossi, G.P. et al. A prospective study of the prevalence of primary aldosteronism in 1,125 hypertensive patients. J. Am. Coll. Cardiol. 48, 2293–2300 (2006).
Conn, J.W. Presidential address. I. Painting background. II. Primary aldosteronism, a new clinical syndrome. J. Lab. Clin. Med. 45, 3–17 (1955).
Scholl, U.I. et al. Hypertension with or without adrenal hyperplasia due to different inherited mutations in the potassium channel KCNJ5. Proc. Natl. Acad. Sci. USA 109, 2533–2538 (2012).
Tadjine, M., Lampron, A., Ouadi, L. & Bourdeau, I. Frequent mutations of β-catenin gene in sporadic secreting adrenocortical adenomas. Clin. Endocrinol. 68, 264–270 (2008).
Catterall, W.A. Signaling complexes of voltage-gated sodium and calcium channels. Neurosci. Lett. 486, 107–116 (2010).
Baig, S.M. et al. Loss of Cav1.3 (CACNA1D) function in a human channelopathy with bradycardia and congenital deafness. Nat. Neurosci. 14, 77–84 (2011).
Hu, C., Rusin, C.G., Tan, Z., Guagliardo, N.A. & Barrett, P.Q. Zona glomerulosa cells of the mouse adrenal cortex are intrinsic electrical oscillators. J. Clin. Invest. 122, 2046–2053 (2012).
Tadross, M.R., Ben Johny, M. & Yue, D.T. Molecular endpoints of Ca2+/calmodulin- and voltage-dependent inactivation of Cav1.3 channels. J. Gen. Physiol. 135, 197–215 (2010).
Clark, V.E. et al. Genomic analysis of non-NF2 meningiomas reveals mutations in TRAF7, KLF4, AKT1 and SMO. Science 339, 1077–1080 (2013).
Platzer, J. et al. Congenital deafness and sinoatrial node dysfunction in mice lacking class D L-type Ca2+ channels. Cell 102, 89–97 (2000).
Hering, S. et al. Pore stability and gating in voltage-activated calcium channels. Channels 2, 61–69 (2008).
Hoda, J.C., Zaghetto, F., Koschak, A. & Striessnig, J. Congenital stationary night blindness type 2 mutations S229P, G369D, L1068P, and W1440X alter channel gating or functional expression of Cav1.4 L-type Ca2+ channels. J. Neurosci. 25, 252–259 (2005).
Hemara-Wahanui, A. et al. A CACNA1F mutation identified in an X-linked retinal disorder shifts the voltage dependence of Cav1.4 channel activation. Proc. Natl. Acad. Sci. USA 102, 7553–7558 (2005).
Splawski, I. et al. Severe arrhythmia disorder caused by cardiac L-type calcium channel mutations. Proc. Natl. Acad. Sci. USA 102, 8089–8096 (2005).
Hans, M. et al. Functional consequences of mutations in the human α1A calcium channel subunit linked to familial hemiplegic migraine. J. Neurosci. 19, 1610–1619 (1999).
Battistini, S. et al. A new CACNA1A gene mutation in acetazolamide-responsive familial hemiplegic migraine and ataxia. Neurology 53, 38–43 (1999).
Scholl, U.I. & Lifton, R.P. New insights into aldosterone-producing adenomas and hereditary aldosteronism: mutations in the K+ channel KCNJ5. Curr. Opin. Nephrol. Hypertens. 22, 141–147 (2013).
Beuschlein, F. et al. Somatic mutations in ATP1A1 and ATP2B3 lead to aldosterone-producing adenomas and secondary hypertension. Nat. Genet. 45, 440–444 (2013).
Kang, S. et al. Cav1.3-selective L-type calcium channel antagonists as potential new therapeutics for Parkinson's disease. Nat. Commun. 3, 1146 (2012).
Peloquin, J.B., Rehak, R., Doering, C.J. & McRory, J.E. Functional analysis of congenital stationary night blindness type-2 CACNA1F mutations F742C, G1007R, and R1049W. Neuroscience 150, 335–345 (2007).
Ducros, A. et al. The clinical spectrum of familial hemiplegic migraine associated with mutations in a neuronal calcium channel. N. Engl. J. Med. 345, 17–24 (2001).
Brenner, C.H. A note on paternity computation in cases lacking a mother. Transfusion 33, 51–54 (1993).
Butler, J.M., Schoske, R., Vallone, P.M., Redman, J.W. & Kline, M.C. Allele frequencies for 15 autosomal STR loci on U.S. Caucasian, African American, and Hispanic populations. J. Forensic Sci. 48, 908–911 (2003).
Gonzalez-Gutierrez, G. et al. The guanylate kinase domain of the β-subunit of voltage-gated calcium channels suffices to modulate gating. Proc. Natl. Acad. Sci. USA 105, 14198–14203 (2008).
Marcantoni, A. et al. Loss of Cav1.3 channels reveals the critical role of L-type and BK channel coupling in pacemaking mouse adrenal chromaffin cells. J. Neurosci. 30, 491–504 (2010).
Roberts-Crowley, M.L. & Rittenhouse, A.R. Arachidonic acid inhibition of L-type calcium (Cav1.3b) channels varies with accessory Cavβ subunits. J. Gen. Physiol. 133, 387–403 (2009).
Acknowledgements
We thank the subjects and families whose participation made this study possible. We thank the staff of the Yale West Campus Genomics Center, the Yale Cellular and Molecular Physiology Microscopy and Imaging Core, the Endocrine Surgical Laboratory, Clinical Research Centre, Uppsala University Hospital and the Department of Surgery, Montefiore Medical Center and Albert Einstein College of Medicine for their invaluable contributions to this research. J. Matthes (Universität Köln) and F. Lehmann-Horn (Universität Ulm) kindly provided us with clones for the α2δ subunits. This work was supported by the US National Institutes of Health (NIH) Centers for Mendelian Genomics (5U54HG006504), the Fondation Leducq Transatlantic Network in Hypertension and the Deutsche Forschungsgemeinschaft and by the Swedish Cancer Society, the Swedish Research Council and the Lions Cancer Fund, Uppsala. G.G. is supported by the Agency for Science, Technology and Research, Singapore. T.C. is a Doris Duke-Damon Runyon Clinical Investigator. R.P.L. is an Investigator of the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Contributions
A.L.F., L.F.S., J.W.K., M.L.P., E.A.H., N.M., M.R.B., T.B., J.R.S., E.L., U.I.S., R.P.L., S.K.L., P. Hellman, G.W., G.Å., P.B. and T.C. ascertained and recruited subjects and obtained samples and medical records. A.L.F., R.K., L.F.S., U.I.S., P.B. and C.N.-W. prepared DNA and RNA samples and maintained sample archives. J.D.O. and S.M. performed exome sequencing. U.I.S. and G.G. performed and analyzed targeted DNA and RNA sequencing. G.G., M.C. and R.P.L. analyzed exome sequencing results. U.I.S. and R.K. performed immunohistochemistry. U.I.S., G.S., R.C.d.O., C.F. and P. Hidalgo made constructs and performed and analyzed electrophysiology. U.I.S., G.G., G.S., C.F., P. Hidalgo and R.P.L. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–5, Supplementary Tables 1–6 and Supplementary Note (PDF 532 kb)
Rights and permissions
About this article
Cite this article
Scholl, U., Goh, G., Stölting, G. et al. Somatic and germline CACNA1D calcium channel mutations in aldosterone-producing adenomas and primary aldosteronism. Nat Genet 45, 1050–1054 (2013). https://doi.org/10.1038/ng.2695
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng.2695
This article is cited by
-
Pathogenicity of de novo CACNA1D Ca2+ channel variants predicted from sequence co-variation
European Journal of Human Genetics (2024)
-
Primary aldosteronism: molecular medicine meets public health
Nature Reviews Nephrology (2023)
-
Somatic SLC30A1 mutations altering zinc transporter ZnT1 cause aldosterone-producing adenomas and primary aldosteronism
Nature Genetics (2023)
-
Identifying primary aldosteronism patients who require adrenal venous sampling: a multi-center study
Scientific Reports (2023)