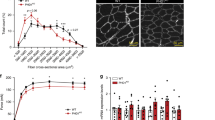
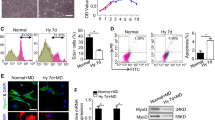
Abstract
HIF prolyl hydroxylases (PHD1–3) are oxygen sensors that regulate the stability of the hypoxia-inducible factors (HIFs) in an oxygen-dependent manner. Here, we show that loss of Phd1 lowers oxygen consumption in skeletal muscle by reprogramming glucose metabolism from oxidative to more anaerobic ATP production through activation of a Pparα pathway. This metabolic adaptation to oxygen conservation impairs oxidative muscle performance in healthy conditions, but it provides acute protection of myofibers against lethal ischemia. Hypoxia tolerance is not due to HIF-dependent angiogenesis, erythropoiesis or vasodilation, but rather to reduced generation of oxidative stress, which allows Phd1-deficient myofibers to preserve mitochondrial respiration. Hypoxia tolerance relies primarily on Hif-2α and was not observed in heterozygous Phd2-deficient or homozygous Phd3-deficient mice. Of medical importance, conditional knockdown of Phd1 also rapidly induces hypoxia tolerance. These findings delineate a new role of Phd1 in hypoxia tolerance and offer new treatment perspectives for disorders characterized by oxidative stress.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Ramirez, J.M., Folkow, L.P. & Blix, A.S. Hypoxia tolerance in mammals and birds: from the wilderness to the clinic. Annu. Rev. Physiol. 69, 113–143 (2007).
Andrews, M.T. Genes controlling the metabolic switch in hibernating mammals. Biochem. Soc. Trans. 32, 1021–1024 (2004).
Kim, J.W., Tchernyshyov, I., Semenza, G.L. & Dang, C.V. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 3, 177–185 (2006).
Papandreou, I., Cairns, R.A., Fontana, L., Lim, A.L. & Denko, N.C. HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell Metab. 3, 187–197 (2006).
Sugden, M.C. & Holness, M.J. Mechanisms underlying regulation of the expression and activities of the mammalian pyruvate dehydrogenase kinases. Arch. Physiol. Biochem. 112, 139–149 (2006).
Bruick, R.K. & McKnight, S.L. A conserved family of prolyl-4-hydroxylases that modify HIF. Science 294, 1337–1340 (2001).
Epstein, A.C. et al. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell 107, 43–54 (2001).
Appelhoff, R.J. et al. Differential function of the prolyl hydroxylases PHD1, PHD2, and PHD3 in the regulation of hypoxia-inducible factor. J. Biol. Chem. 279, 38458–38465 (2004).
Berra, E. et al. HIF prolyl-hydroxylase 2 is the key oxygen sensor setting low steady-state levels of HIF-1alpha in normoxia. EMBO J. 22, 4082–4090 (2003).
Elson, D.A. et al. Induction of hypervascularity without leakage or inflammation in transgenic mice overexpressing hypoxia-inducible factor-1α. Genes Dev. 15, 2520–2532 (2001).
Willam, C. et al. Peptide blockade of HIFα degradation modulates cellular metabolism and angiogenesis. Proc. Natl. Acad. Sci. USA 99, 10423–10428 (2002).
Takeda, K. et al. Placental but not heart defects are associated with elevated hypoxia-inducible factor α levels in mice lacking prolyl hydroxylase domain protein 2. Mol. Cell. Biol. 26, 8336–8346 (2006).
Hochachka, P.W. Mechanism and evolution of hypoxia-tolerance in humans. J. Exp. Biol. 201, 1243–1254 (1998).
Schuler, M. et al. PGC1α expression is controlled in skeletal muscles by PPARβ, whose ablation results in fiber-type switching, obesity, and type 2 diabetes. Cell Metab. 4, 407–414 (2006).
Kotani, K., Peroni, O.D., Minokoshi, Y., Boss, O. & Kahn, B.B. GLUT4 glucose transporter deficiency increases hepatic lipid production and peripheral lipid utilization. J. Clin. Invest. 114, 1666–1675 (2004).
Mason, S.D. et al. Loss of skeletal muscle HIF-1α results in altered exercise endurance. PLoS Biol. 2, e288 (2004).
Sloniger, M.A., Cureton, K.J., Prior, B.M. & Evans, E.M. Lower extremity muscle activation during horizontal and uphill running. J. Appl. Physiol. 83, 2073–2079 (1997).
Finck, B.N. et al. A potential link between muscle peroxisome proliferator-activated receptor-α signaling and obesity-related diabetes. Cell Metab. 1, 133–144 (2005).
Jeoung, N.H. et al. Role of pyruvate dehydrogenase kinase isoenzyme 4 (PDHK4) in glucose homeostasis during starvation. Biochem. J. 397, 417–425 (2006).
Harris, R.A., Bowker-Kinley, M.M., Huang, B. & Wu, P. Regulation of the activity of the pyruvate dehydrogenase complex. Adv. Enzyme Regul. 42, 249–259 (2002).
Zaccagnini, G. et al. p66ShcA modulates tissue response to hindlimb ischemia. Circulation 109, 2917–2923 (2004).
Bracken, C.P., Whitelaw, M.L. & Peet, D.J. The hypoxia-inducible factors: key transcriptional regulators of hypoxic responses. Cell. Mol. Life Sci. 60, 1376–1393 (2003).
Doege, K., Heine, S., Jensen, I., Jelkmann, W. & Metzen, E. Inhibition of mitochondrial respiration elevates oxygen concentration but leaves regulation of hypoxia-inducible factor (HIF) intact. Blood 106, 2311–2317 (2005).
Clanton, T.L. Hypoxia-induced reactive oxygen species formation in skeletal muscle. J. Appl. Physiol. 102, 2379–2388 (2007).
Fiskum, G. et al. Protection against ischemic brain injury by inhibition of mitochondrial oxidative stress. J. Bioenerg. Biomembr. 36, 347–352 (2004).
Imai, H., Graham, D.I., Masayasu, H. & Macrae, I.M. Antioxidant ebselen reduces oxidative damage in focal cerebral ischemia. Free Radic. Biol. Med. 34, 56–63 (2003).
Ott, M., Gogvadze, V., Orrenius, S. & Zhivotovsky, B. Mitochondria, oxidative stress and cell death. Apoptosis 12, 913–922 (2007).
Gardner, P.R. Aconitase: sensitive target and measure of superoxide. Methods Enzymol. 349, 9–23 (2002).
Wu, P., Peters, J.M. & Harris, R.A. Adaptive increase in pyruvate dehydrogenase kinase 4 during starvation is mediated by peroxisome proliferator-activated receptor alpha. Biochem. Biophys. Res. Commun. 287, 391–396 (2001).
Scortegagna, M. et al. Multiple organ pathology, metabolic abnormalities and impaired homeostasis of reactive oxygen species in Epas1−/− mice. Nat. Genet. 35, 331–340 (2003).
Brusselmans, K. et al. Heterozygous deficiency of hypoxia-inducible factor-2α protects mice against pulmonary hypertension and right ventricular dysfunction during prolonged hypoxia. J. Clin. Invest. 111, 1519–1527 (2003).
Sugden, M.C. & Holness, M.J. Recent advances in mechanisms regulating glucose oxidation at the level of the pyruvate dehydrogenase complex by PDKs. Am. J. Physiol. Endocrinol. Metab. 284, E855–E862 (2003).
Becker, L.B., vanden Hoek, T.L., Shao, Z.H., Li, C.Q. & Schumacker, P.T. Generation of superoxide in cardiomyocytes during ischemia before reperfusion. Am. J. Physiol. 277, H2240–H2246 (1999).
Magalhaes, J. et al. Acute and severe hypobaric hypoxia increases oxidative stress and impairs mitochondrial function in mouse skeletal muscle. J. Appl. Physiol. 99, 1247–1253 (2005).
Thomas, S. & Fell, D.A. A control analysis exploration of the role of ATP utilisation in glycolytic-flux control and glycolytic-metabolite-concentration regulation. Eur. J. Biochem. 258, 956–967 (1998).
Lefebvre, P., Chinetti, G., Fruchart, J.C. & Staels, B. Sorting out the roles of PPARα in energy metabolism and vascular homeostasis. J. Clin. Invest. 116, 571–580 (2006).
Haddad, G.G. Tolerance to low O2: lessons from invertebrate genetic models. Exp. Physiol. 91, 277–282 (2006).
Buck, M.J., Squire, T.L. & Andrews, M.T. Coordinate expression of the PDK4 gene: a means of regulating fuel selection in a hibernating mammal. Physiol. Genomics 8, 5–13 (2002).
Semenza, G.L. Targeting HIF-1 for cancer therapy. Nat. Rev. Cancer 3, 721–732 (2003).
Wouters, B.G., van den Beucken, T., Magagnin, M.G., Lambin, P. & Koumenis, C. Targeting hypoxia tolerance in cancer. Drug Resist. Updat. 7, 25–40 (2004).
Balaban, R.S., Nemoto, S. & Finkel, T. Mitochondria, oxidants, and aging. Cell 120, 483–495 (2005).
Wallace, D.C. Mitochondrial diseases in man and mouse. Science 283, 1482–1488 (1999).
Luttun, A. et al. Revascularization of ischemic tissues by PlGF treatment, and inhibition of tumor angiogenesis, arthritis and atherosclerosis by anti-Flt1. Nat. Med. 8, 831–840 (2002).
Jordan, B.F. et al. Insulin increases the sensitivity of tumors to irradiation: involvement of an increase in tumor oxygenation mediated by a nitric oxide-dependent decrease of the tumor cells oxygen consumption. Cancer Res. 62, 3555–3561 (2002).
Buyse, J. et al. Energy and protein metabolism between 3 and 6 weeks of age of male broiler chickens selected for growth rate or for improved food efficiency. Br. Poult. Sci. 39, 264–272 (1998).
Kuznetsov, A.V. et al. Mitochondrial defects and heterogeneous cytochrome c release after cardiac cold ischemia and reperfusion. Am. J. Physiol. Heart Circ. Physiol. 286, H1633–H1641 (2004).
Gnaiger, E. Oxygen conformance of cellular respiration. A perspective of mitochondrial physiology. Adv. Exp. Med. Biol. 543, 39–55 (2003).
Boushel, R. et al. Patients with type 2 diabetes have normal mitochondrial function in skeletal muscle. Diabetologia 50, 790–796 (2007).
McMahon, J.M., Signori, E., Wells, K.E., Fazio, V.M. & Wells, D.J. Optimisation of electrotransfer of plasmid into skeletal muscle by pretreatment with hyaluronidase–increased expression with reduced muscle damage. Gene Ther. 8, 1264–1270 (2001).
Acknowledgements
J.A. is sponsored by the Centro Nacional de Investigaciones Cardiovasculares (CNIC; Spain) and Flanders Institute of Biotechnology (VIB; Belgium); M.S. by the Deutsche Forschungsgemeinschaft (Germany) and the Lymphatic Research Foundation; K.V.G. by a doctoral fellowship from the K.U. Leuven; P.F. by a postdoctoral fellowship from the K.U. Leuven; and H.L. by a postdoctoral fellowship from Fond Québécois de la nature et des technologies. This work is supported, in part, by grant GOA2006/11/KULeuven from the University of Leuven, Belgium, grant IUAP05/02 from the Federal Government Belgium, grants FWO G.0265 and FWO G.0387 from the Flanders Research Foundation, Belgium, grant 5RO1GM037704 from the US National Institutes of Health (to F.L.), grant 12RO1DK47844 from the US National Institutes of Health (to R.A.H.), grant 2.4532.03 from the Belgian Fonds National de la Recherche Scientifique (to F.S.), and a grant from the Molecular Small Animal Imaging Center, University of Leuven (to L.M. and P.V.H.) and by a Programme Grant from the British Heart Foundation (to P.H.M. and P.J.R.). The authors thank A. Bouche, A. Carton, P. Chevron, M. De Mol, E. Gils, B. Hermans, L. Kieckens, W.Y. Man, W. Martens, A. Manderveld, E. Meyhi, L. Notebaert, J. Souffreau, C. Vanhuylebroeck, B. Vanwetswinkel, P. Van Wesemael, Q. Swennen, B. Kamers and S. Wyns for their contributions.
Author information
Authors and Affiliations
Contributions
J.A.: analysis of all data; design and performance of NMR spectroscopy, assays of metabolic enzymes, metabolites, indirect calorimetry, RNA and protein expression, and respiration studies; writing of the paper. M.S.: analysis of all data; design and performance of running tests, hind limb vascularization, EPR, respiration studies, indirect calorimetry and histological analysis; writing of the paper. K.V.G.: analysis of all data; design and performance of RNA interference experiments, metabolic assays, indirect calorimetry and RNA and protein expression; writing of the paper. P.F.: analysis of all data; design and performance of metabolic assays and mitochondrial respiration studies; writing of the paper. T.D. and P.V.H.: NMR spectroscopy. M.M., P.L., S.K.H., A.G., S.Z. and T.B.: protein expression analysis, cell culture experiments, mitochondrial performance assays. N.H.J., F.G., R.A.H. and R.D.: metabolic enzyme and metabolite assays; oxidative stress assays. D.L. and M.D.: generation of PHD1-deficient mice; genetic analysis of background; statistical analysis. A.D.-J. and T.V.: design of constructs expressing interference short hairpin RNAs. P.V.N., K.D.B. and P.H.: muscle exercise studies. C.W. and C.P.: design and construction of PHD1 targeting vector. M.T. and L. Moons.: histological analysis. R.N. and F. Sluse.: mitochondrial respiration studies. C.D., B.W., J.N. and L. Mortelmans: glucose metabolism studies. B.J. and B.G.: EPR studies. R.S.-M. and F.L.: mitochondrial ultrastructure. P.S.: micro-CT analysis. J.B.: indirect calorimetry. H.L. and E.G.: analysis and performance of mitochondrial respiration studies in isolated myofibers. P.V.V., F. Schuit and M.B.: analysis and design of metabolic experiments and oxidative stress assays; writing of the paper. P.R., P.M and P.C.: scientific direction; generation of PHD knockout mice; conception of hypoxia tolerance studies; design of experimental approaches; analysis of data; writing of the paper.
Corresponding author
Ethics declarations
Competing interests
P.H.M. is a director, consultant and equity holder in ReOx Ltd. P.J.R., P.H.M and C.W.P. are founding scientists of ReOx Ltd.
Supplementary information
Supplementary Text and Figures
Supplementary Note, Supplementary Table 1, Supplementary Figure 1 (PDF 680 kb)
Rights and permissions
About this article
Cite this article
Aragonés, J., Schneider, M., Van Geyte, K. et al. Deficiency or inhibition of oxygen sensor Phd1 induces hypoxia tolerance by reprogramming basal metabolism. Nat Genet 40, 170–180 (2008). https://doi.org/10.1038/ng.2007.62
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng.2007.62
This article is cited by
-
PHD1-3 oxygen sensors in vivo—lessons learned from gene deletions
Pflügers Archiv - European Journal of Physiology (2024)
-
Targeting hypoxia-inducible factors: therapeutic opportunities and challenges
Nature Reviews Drug Discovery (2023)
-
Recent Advances and Therapeutic Implications of 2-Oxoglutarate-Dependent Dioxygenases in Ischemic Stroke
Molecular Neurobiology (2023)
-
Histone H3 proline 16 hydroxylation regulates mammalian gene expression
Nature Genetics (2022)
-
Gill and liver transcriptomic responses of Achirus lineatus (Neopterygii: Achiridae) exposed to water-accommodated fraction (WAF) of light crude oil reveal an onset of hypoxia-like condition
Environmental Science and Pollution Research (2021)