Abstract
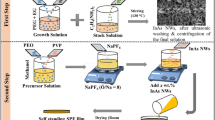
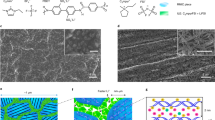
In contrast to conventional organic liquid electrolytes that have leakage, flammability and chemical stability issues, solid electrolytes are widely considered as a promising candidate for the development of next-generation safe lithium-ion batteries. In solid polymer electrolytes that contain polymers and lithium salts, inorganic nanoparticles are often used as fillers to improve electrochemical performance, structure stability, and mechanical strength. However, such composite polymer electrolytes generally have low ionic conductivity. Here we report that a composite polymer electrolyte with well-aligned inorganic Li+-conductive nanowires exhibits an ionic conductivity of 6.05 × 10−5 S cm-1 at 30 ∘C, which is one order of magnitude higher than previous polymer electrolytes with randomly aligned nanowires. The large conductivity enhancement is ascribed to a fast ion-conducting pathway without crossing junctions on the surfaces of the aligned nanowires. Moreover, the long-term structural stability of the polymer electrolyte is also improved by the use of nanowires.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Quartarone, E. & Mustarelli, P. Electrolytes for solid-state lithium rechargeable batteries: recent advances and perspectives. Chem. Soc. Rev. 40, 2525–2540 (2011).
Stephan, A. M. & Nahm, K. S. Review on composite polymer electrolytes for lithium batteries. Polymer 47, 5952–5964 (2006).
Zheng, G. et al. Interconnected hollow carbon nanospheres for stable lithium metal anodes. Nat. Nanotech. 9, 618–623 (2014).
Bouchet, R. et al. Single-ion BAB triblock copolymers as highly efficient electrolytes for lithium-metal batteries. Nat. Mater. 12, 452–457 (2013).
Denoyel, R., Armand, M. & Fergus, J. W. Ceramic and polymeric solid electrolytes for lithium-ion batteries. J. Power Sources 195, 4554–4569 (2010).
Armand, M. & Tarascon, J.-M. Building better batteries. Nature 451, 652–657 (2008).
Aricò, A. S., Bruce, P., Scrosati, B., Tarascon, J.-M. & van Schalkwijk, W. Nanostructured materials for advanced energy conversion and storage devices. Nat. Mater. 4, 366–377 (2005).
Cao, C., Li, Z., Wang, X. L., Zhao, X. & Han, W. Q. Recent advances in inorganic solid electrolytes for lithium batteries. Front. Energy Res. 2, 25 (2014).
Oudenhoven, J. F. M., Baggetto, L. & Notten, P. H. L. All-solid-state lithium-ion microbatteries: a review of various three-dimensional concepts. Adv. Energy Mater. 1, 10–33 (2011).
Agrawal, R. C. & Pandey, G. P. Solid polymer electrolytes: materials designing and all-solid-state battery applications: an overview. J. Phys. D 41, 223001–223018 (2008).
Mizuno, F., Hayashi, A., Tadanaga, K. & Tatsumisago, M. New, highly ion-conductive crystals precipitated from Li2S-P2S5 glasses. Adv. Mater. 17, 918–921 (2005).
Minami, K., Hayashi, A. & Tatsumisago, M. Preparation and characterization of superionic conducting Li7P3S11 crystal from glassy liquids. J. Ceram. Soc. Jpn 118, 305–308 (2010).
Kamaya, N. et al. A lithium superionic conductor. A lithium superionic conductor. Nat. Mater. 10, 682–686 (2011).
Kawai, H. & Kuwano, J. Lithium ion conductivity of a-site deficient perovskite solid solution La0.67−xLi3xTiO3 . J. Electrochem. Soc. 141, L78–L79 (1994).
Zhu, Y., Zhang, Y. & Lu, L. Influence of crystallization temperature on ionic conductivity of lithium aluminum germanium phosphate glass-ceramic. J. Power Sources 290, 123–129 (2015).
Wang, Y. & Zhong, W. H. Development of electrolytes towards achieving safe and high performance energy storage devices: a review. ChemElectroChem 2, 22–36 (2015).
Croce, F., Appetecchi, G. B., Persi, L. & Scrosati, B. Nanocomposite polymer electrolytes for lithium batteries. Nature 394, 456–458 (1998).
Hsu, C. Y. et al. High thermal and electrochemical stability of PVDF-graft-PAN copolymer hybrid PEO membrane for safety reinforced lithium-ion battery. RSC Adv. 6, 18082–18088 (2016).
Wang, S. H. et al. Design of poly (acrylonitrile)-based gel electrolytes for high-performance lithium ion batteries. ACS Appl. Mater. Inter. 6, 19360–19370 (2014).
Hong, H. et al. Studies on PAN-based lithium salt complex. Electrochim. Acta 37, 1671–1673 (1992).
Qian, X. et al. Impedance study of (PEO)10LiClO4-Al2O3 composite polymer electrolyte with blocking electrodes. Electrochim. Acta 46, 1829–1836 (2001).
Kim, J. W., Ji, K. S., Lee, J. P. & Park, J. W. Electrochemical characteristics of two types of PEO-based composite electrolyte with functional SiO2 . J. Power Sources 119–121, 415–421 (2003).
Balazs, A. C., Emrick, T. & Russell, T. P. Nanoparticle polymer composites: where two small worlds meet. Science 314, 1107–1110 (2006).
Choi, J. H. et al. Enhancement of ionic conductivity of composite membranes for all-solid-state lithium rechargeable batteries incorporating tetragonal Li7La3Zr2O12 into a polyethylene oxide matrix. J. Power Sources 274, 458–463 (2015).
Wieczorek, W., Such, K., Wyciślik, H. & Płocharski, J. Modifications of crystalline structure of PEO polymer electrolytes with ceramic additives. Solid State Ion. 36, 255–257 (1989).
Wieczorek, W., Florjancyk, Z. & Stevens, J. R. Composite polyether based solid electrolytes. Electrochim. Acta 40, 2251–2258 (1995).
Hu, Y.-S. Getting solid. Nat. Energy 1, 16042 (2016).
Weston, J. E. & Steele, B. C. H. Effects of inert fillers on the mechanical and electrochemical properties of lithium salt-poly (ethylene oxide) polymer electrolytes. Solid State Ion. 7, 75–79 (1982).
Capuano, F., Croce, F. & Scrosati, B. Composite polymer electrolytes. J. Electrochem. Soc. 138, 1918–1922 (1991).
Chiang, C. Y., Reddy, M. J. & Chu, P. P. Nano-tube TiO2 composite PVdF/LiPF6 solid membranes. Solid State Ion. 175, 631–635 (2004).
Wagemaker, M. et al. Multiple Li positions inside oxygen octahedra in lithiated TiO2 anatase. J. Am. Chem. Soc. 125, 840–848 (2003).
Chu, P. P., Reddy, M. J. & Kao, H. M. Novel composite polymer electrolyte comprising mesoporous structured SiO2 and PEO/Li. Solid State Ion. 156, 141–153 (2003).
Croce, F. et al. Physical and chemical properties of nanocomposite polymer electrolytes. J. Phys. Chem. B 103, 10632–10638 (1999).
Croce, F. et al. Role of the ceramic fillers in enhancing the transport properties of composite polymer electrolytes. Electrochim. Acta 46, 2457–2461 (2001).
Quartarone, E., Mustarelli, P. & Magistris, A. PEO-based composite polymer electrolytes. Solid State Ion. 110, 1–14 (1998).
Scrosati, B., Croce, F. & Persi, L. Impedance spectroscopy study of PEO-based nanocomposite polymer electrolytes. J. Electrochem. Soc. 147, 1718–1721 (2000).
Liu, W. et al. Ionic conductivity enhancement of polymer electrolytes with ceramic nanowires fillers. Nano Lett. 15, 2740–2745 (2015).
Cetiner, S. et al. Polymerization of pyrrole derivatives on polyacrylonitrile matrix, FTIR–ATR and dielectric spectroscopic characterization of composite thin films. Synth. Met. 160, 1189–1196 (2010).
Phadke, M. A. et al. Poly (acrylonitrile) ultrafiltration membranes. I. Polymer-salt-solvent interactions. J. Polymer Sci. B 43, 2061–2073 (2005).
Funke, K. Jump relaxation in solid electrolytes. Prog. Solid State Chem. 22, 111–195 (1993).
Osman, Z. et al. AC ionic conductivity and DC polarization method of lithium ion transport in PMMA-LiBF4 gel polymer electrolytes. Results Phys. 2, 1–4 (2012).
Neudecker, B. J. & Weppner, W. Li9SiAlO8: a lithium ion electrolyte for voltages above 5.4 V. J. Electrochem. Soc. 143, 2198–2203 (1996).
Kanno, R. et al. Synthesis of a new lithium ionic conductor, thio-LISICON–lithium germanium sulfide system. Solid State Ion. 130, 97–104 (2000).
Scrosati, B., Croce, F. & Panero, S. Progress in lithium polymer battery R&D. J. Power Sources 100, 93–100 (2001).
Lin, C. W. et al. Influence of TiO2 nano-particles on the transport properties of composite polymer electrolyte for lithium-ion batteries. J. Power Sources 146, 397–401 (2005).
Sun, H. Y. et al. Enhanced lithium-ion transport in PEO-based composite polymer electrolytes with ferroelectric BaTiO3 . J. Electrochem. Soc. 146, 1672–1676 (1999).
Evans, J., Vincent, C. A. & Bruce, P. G. Electrochemical measurement of transference numbers in polymer electrolytes. Polymer 28, 2324–2328 (1987).
Hodge, I. M., Ingram, M. D. & West, A. R. Impedance and modulus spectroscopy of polycrystalline solid electrolytes. J. Electroanal. Chem. 74, 125–143 (1976).
Cantwell, P. R. et al. Grain boundary complexions. Acta Mater. 62, 1–48 (2014).
Zhou, Z. et al. Development of carbon nanofibers from aligned electrospun polyacrylonitrile nanofiber bundles and characterization of their microstructural, electrical, and mechanical properties. Polymer 50, 2999–3006 (2009).
Wieczorek, W., Stevens, J. R. & Florjańczyk, Z. Composite polyether based solid electrolytes. The Lewis acid-base approach. Solid State Ion. 85, 67–72 (1996).
Nan, C. W., Fan, L., Lin, Y. & Cai, Q. Enhanced ionic conductivity of polymer electrolytes containing nanocomposite SiO2 particles. Phys. Rev. Lett. 91, 266104 (2003).
Gowneni, S., Ramanjaneyulu, K. & Basak, P. Polymer-nanocomposite brush-like architectures as an all-solid electrolyte matrix. ACS Nano 8, 11409–11424 (2014).
Sata, N. et al. Mesoscopic fast ion conduction in nanometre-scale planar heterostructures. Nature 408, 946–949 (2000).
Lubben, D. & Modine, F. A. Enhanced ionic conduction mechanisms at LiI/Al2O3 interfaces. J. Appl. Phys. 80, 5150–5157 (1996).
Liu, W., Lin, D., Sun, J., Zhou, G. & Cui, Y. Improved lithium ionic conductivity in composite polymer electrolytes with oxide-ion conducting nanowires. ACS Nano 10, 11407–11413 (2016).
Acknowledgements
This work is supported by Samsung Electronics.
Author information
Authors and Affiliations
Contributions
W.L. and Y.C. conceived the experiment and carried out data analysis. W.L. performed materials fabrication and characterization. S.W.L. performed the numerical simulation. D.L., F.S. and S.W. assisted in experimental work. A.D.S. assisted in language editing. W.L. and Y.C. wrote the paper. All the authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Figures 1–13, Supplementary Table 1, Supplementary Notes, Supplementary References. (PDF 1349 kb)
Rights and permissions
About this article
Cite this article
Liu, W., Lee, S., Lin, D. et al. Enhancing ionic conductivity in composite polymer electrolytes with well-aligned ceramic nanowires. Nat Energy 2, 17035 (2017). https://doi.org/10.1038/nenergy.2017.35
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/nenergy.2017.35
This article is cited by
-
Quasi-solid-state plasticized chitosan biopolymer electrolyte with enhanced Mg2+ ion mobility for next-generation Mg ion battery
Journal of Solid State Electrochemistry (2024)
-
Inorganic lithium-ion conductors for fast-charging lithium batteries: a review
Journal of Solid State Electrochemistry (2024)
-
Enhanced 3D framework composite solid electrolyte with alumina-modified Li1.4Al0.4Ti1.6(PO4)3 for solid-state lithium battery
Ionics (2024)
-
Polyethylene Oxide-Based Composite Solid Electrolytes for Lithium Batteries: Current Progress, Low-Temperature and High-Voltage Limitations, and Prospects
Electrochemical Energy Reviews (2024)
-
From Liquid to Solid-State Lithium Metal Batteries: Fundamental Issues and Recent Developments
Nano-Micro Letters (2024)