Abstract
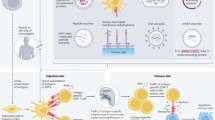
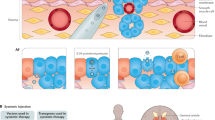
The lack of effective therapies for advanced prostate cancer mandates continued development of alternative treatment strategies. Insights into the regulation of immune responses and the malignant process have facilitated the emergence of new immune-based strategies, currently under investigation in clinical trials. Like other forms of targeted therapy, cancer vaccines hold the promise of achieving cancer control without inducing overt toxicity. Many prostate cancer vaccines at different phases of development have been tested in clinical trials. Vaccination strategies under consideration include: immunization with defined antigenic preparations such as synthetic peptides, proteins or plasmid DNA; antigen-loaded dendritic cells; manipulated tumor cells; or with viral vectors engineered to express immunogenic genes. Although the underlying mechanisms of immunization may vary, all strategies share the common goal of eliciting immune responses against prostate tumor-associated antigens or of enhancing an otherwise weak antitumor response in the cancer patient. Unlocking the therapeutic potential of cancer vaccines will require a thorough understanding of cellular and molecular mechanisms that modulate the immune response. In this review, we provide an overview of vaccine-based strategies for prostate cancer therapy, discuss their mechanisms of action, and provide relevant clinical trial data.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Ries LAG et al. (Eds; 2002) SEER Cancer Statistics Review 1973–1999. Bethesda: National Cancer Institute [http://seer.cancer.gov/csr/1973_1999/sections.html]
Vieweg J and Dannull J (2004, in press) Future perspectives: Immunotherapy and vaccines in prostate cancer. In Management of Prostate Cancer. 367–392 (Ed Cummings KB) New York: Marcel Dekker
Correale P et al. (1997) In vitro generation of human cytotoxic T lymphocytes specific for peptides derived from prostate-specific antigen. J Natl Cancer Inst 89: 293–300
Murphy G et al. (1996) Phase I clinical trial: T-cell therapy for prostate cancer using autologous dendritic cells pulsed with HLA-A0201-specific peptides from prostate-specific membrane antigen. Prostate 29: 371–380
Disis ML and Schiffman K (2001) Cancer vaccines targeting the HER2/neu oncogenic protein. Semin Oncol 28: 12–20
Woll MM et al. (2004) Direct measurement of peptide-specific CD8+ T cells using HLA-A2: Ig dimer for monitoring the in vivo immune response to a HER2/neu vaccine in breast and prostate cancer patients. J Clin Immunol 24: 449–461
McNeel DG et al. (2003) Pilot study of an HLA-A2 peptide vaccine using flt3 ligand as a systemic vaccine adjuvant. J Clin Immunol 23: 62–72
Noguchi M et al. (2003) Induction of cellular and humoral immune responses to tumor cells and peptides in HLA-A24 positive hormone-refractory prostate cancer patients by peptide vaccination. Prostate 57: 80–92
Noguchi M et al. (2004) Phase I trial of patient-oriented vaccination in HLA-A2-positive patients with metastatic hormone-refractory prostate cancer. Cancer Sci 95: 77–84
Meidenbauer N et al. (2000) Generation of PSA-reactive effector cells after vaccination with a PSA-based vaccine in patients with prostate cancer. Prostate 43: 88–100
Pardoll DM and Topalian SL (1998) The role of CD4+ T cell responses in antitumor immunity. Curr Opin Immunol 10: 588–594
Ulmer JB et al. (1993) Heterologous protection against influenza by injection of DNA encoding a viral protein. Science 259: 1745–1749
Liu MA (2003) DNA vaccines: a review. J Intern Med 253: 402–410
Pavlenko M et al. (2004) A phase I trial of DNA vaccination with a plasmid expressing prostate-specific antigen in patients with hormone-refractory prostate cancer. Br J Cancer 91: 688–694
Mincheff M et al. (2000) Naked DNA and adenoviral immunizations for immunotherapy of prostate cancer: a phase I/II clinical trial. Eur Urol 38: 208–217
Pardoll DM (2002) Spinning molecular immunology into successful immunotherapy. Nat Rev Immunol 2: 227–238
Eder JP et al. (2000) A phase I trial of a recombinant vaccinia virus expressing prostate-specific antigen in advanced prostate cancer. Clin Cancer Res 6: 1632–1638
Gulley J et al. (2002) Phase I study of a vaccine using recombinant vaccinia virus expressing PSA (rV-PSA) in patients with metastatic androgen-independent prostate cancer. Prostate 53: 109–117
Sanda MG et al. (1999) Recombinant vaccinia-PSA (PROSTVAC) can induce a prostate-specific immune response in androgen-modulated human prostate cancer. Urology 53: 260–266
Kaufman HL et al. (2004) Phase II randomized study of vaccine treatment of advanced prostate cancer (E7897): a trial of the Eastern Cooperative Oncology Group. J Clin Oncol 22: 2122–2132
Banchereau J and Steinman RM (1998) Dendritic cells and the control of immunity. Nature 392: 245–252
Tjoa BA et al. (1999) Follow-up evaluation of a phase II prostate cancer vaccine trial. Prostate 40: 125–129
Hsu FJ et al. (1996) Vaccination of patients with B-cell lymphoma using autologous antigen-pulsed dendritic cells. Nat Med 2: 52–58
Small EJ et al. (2000) Immunotherapy of hormone-refractory prostate cancer with antigen-loaded dendritic cells. J Clin Oncol 18: 3894–3903
Burch PA et al. (2000) Priming tissue-specific cellular immunity in a phase I trial of autologous dendritic cells for prostate cancer. Clin Cancer Res 6: 2175–2182
Burch PA et al. (2004) Immunotherapy (APC8015, Provenge) targeting prostatic acid phosphatase can induce durable remission of metastatic androgen-independent prostate cancer: a Phase 2 trial. Prostate 60: 197–204
Fong L et al. (2001) Dendritic cell-based xenoantigen vaccination for prostate cancer immunotherapy. J Immunol 167: 7150–7156
Fong L et al. (2001) Dendritic cells injected via different routes induce immunity in cancer patients. J Immunol 166: 4254–4259
Fong L et al. (1997) Induction of tissue-specific autoimmune prostatitis with prostatic acid phosphatase immunization: implications for immunotherapy of prostate cancer. J Immunol 159: 3113–3117
Barrou B et al. (2004) Vaccination of prostatectomized prostate cancer patients in biochemical relapse, with autologous dendritic cells pulsed with recombinant human PSA. Cancer Immunol Immunother 53: 453–460
Dhodapkar MV et al. (2001) Antigen-specific inhibition of effector T cell function in humans after injection of immature dendritic cells. J Exp Med 193: 233–238
Gilboa E and Vieweg J (2004) Cancer immunotherapy with mRNA-transfected dendritic cells. Immunol Rev 199: 251–263
Heiser A et al. (2001) Induction of polyclonal prostate cancer specific cytotoxic T lymphocytes using dendritic cells transfected with amplified tumor RNA. J Immunol 166: 2953–2960
Heiser A et al. (2002) Autologous dendritic cells transfected with prostate-specific antigen RNA stimulate CTL responses against metastatic prostate tumors. J Clin Invest 109: 409–417
Shay JW and Bacchetti S (1997) A survey of telomerase activity in human cancer. Eur J Cancer 33: 787–791
Vonderheide RH et al. (2004) Vaccination of cancer patients against telomerase induces functional antitumor CD8+T lymphocytes. Clin Cancer Res 10: 828–839
Su Z et al. (2004) Vaccination of metastatic prostate cancer patients using mature dendritic cells transfected with mRNA encoding hTERT or an MHC class II targeted hTERT/LAMP fusion protein: results from a phase I clinical trial. J Clin Oncol 22 (Suppl; abstract 2507): 164
Simons JW et al. (1999) Induction of immunity to prostate cancer antigens: results of a clinical trial of vaccination with irradiated autologous prostate tumor cells engineered to secrete granulocyte-macrophage colony-stimulating factor using ex vivo gene transfer. Cancer Res 59: 5160–5168
Nelson WG et al. (2000) Cancer cells engineered to secrete granulocyte-macrophage colony-stimulating factor using ex vivo gene transfer as vaccines for the treatment of genitourinary malignancies. Cancer Chemother Pharmacol 46 (Suppl): S67–S72
Simons JW et al. (1997) Bioactivity of autologous irradiated renal cell carcinoma vaccines generated by ex vivo granulocyte-macrophage colony-stimulating factor gene transfer. Cancer Res 57: 1537–1546
Eaton JD et al. (2002) Allogeneic whole-cell vaccine: a phase I/II study in men with hormone-refractory prostate cancer. BJU Int 89: 19–26
Pandha HS et al. (2004) Dendritic cell immunotherapy for urological cancers using cryopreserved allogeneic tumour lysate-pulsed cells: a phase I/II study. BJU Int 94: 412–418
Parry S et al. (2001) Identification of MUC-1 proteolytic cleavage sites in vivo. Biochem Biophys Res Commun 283: 715–720
Agrawal B et al. (1996) In vitro induction of MUC-1 peptide-specific type 1 T lymphocyte and cytotoxic T lymphocyte responses from healthy multiparous donors. J Immunol 157: 2089–2095
Pantuck AJ et al. (2004) Phase I trial of antigen-specific gene therapy using a recombinant vaccinia virus encoding MUC-1 and IL-2 in MUC-1-positive patients with advanced prostate cancer. J Immunother 27: 240–253
Slovin SF et al. (1999) Carbohydrate vaccines in cancer: immunogenicity of a fully synthetic globo H hexasaccharide conjugate in man. Proc Natl Acad Sci USA 96: 5710–5715
Slovin SF et al. (2003) Fully synthetic carbohydrate-based vaccines in biochemically relapsed prostate cancer: clinical trial results with alpha-N-acetylgalactosamine-O-serine/threonine conjugate vaccine. J Clin Oncol 21: 4292–4298
Slovin SF and Scher HI (1999) Peptide and carbohydrate vaccines in relapsed prostate cancer: immunogenicity of synthetic vaccines in man–clinical trials at Memorial Sloan-Kettering Cancer Center. Semin Oncol 26: 448–454
Sutmuller RP et al. (2001) Synergism of cytotoxic T lymphocyte-associated antigen 4 blockade and depletion of CD25+ regulatory T cells in antitumor therapy reveals alternative pathways for suppression of autoreactive cytotoxic T lymphocyte responses. J Exp Med 194: 823–832
Kusmartsev S et al. (2003) All-trans-retinoic acid eliminates immature myeloid cells from tumor-bearing mice and improves the effect of vaccination. Cancer Res 63: 4441–4449
Acknowledgements
Some of the studies referred to in this manuscript were in part supported by grants from the National Cancer Institute (RO1 CA93910, R21 CA098446, RO1 CA89102), and the National Center for Research Resources, (MO1-RR-30) General Clinical Research Centers Program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Dr Vieweg has received research support from the Geron Corporation. He has acted as a consultant for Merix Biosciences, Novartis Pharmaceuticals Corporation and for Glaxosmithkline.
Rights and permissions
About this article
Cite this article
Vieweg, J., Dannull, J. Technology Insight: vaccine therapy for prostate cancer. Nat Rev Urol 2, 44–51 (2005). https://doi.org/10.1038/ncpuro0079
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ncpuro0079
This article is cited by
-
Combining T-cell immunotherapy and anti-androgen therapy for prostate cancer
Prostate Cancer and Prostatic Diseases (2013)
-
Enhancing the efficacy of cancer vaccines in urologic oncology: new directions
Nature Reviews Urology (2009)
-
Transforming growth factor-β-mediated signaling in T lymphocytes impacts on prostate-specific immunity and early prostate tumor progression
Laboratory Investigation (2009)
-
Antitumor activity of a self-adjuvanting glyco-lipopeptide vaccine bearing B cell, CD4+ and CD8+ T cell epitopes
Cancer Immunology, Immunotherapy (2009)
-
Aktive Immuntherapie urologischer Tumoren und tumorbedingte Immunsuppression
Der Urologe (2008)