Abstract
Determining the correct dosage for the majority of traditional chemotherapeutic agents presents a challenge because most drugs have a narrow therapeutic index, which results in a fine balance between doses that cause significant drug toxicity and loss of efficacy. Dosing calculations for most agents use the patient's body surface area, a method that correlates poorly with drug pharmacokinetics. Genetic differences in drug-metabolizing enzymes are being evaluated in an effort to explain the pharmacokinetic and pharmacodynamic variability seen with many chemotherapeutic agents. Elucidation of the underlying reasons for this variability will enable individualization of therapy to minimize toxicity and maximize efficacy, and thus improve control over the narrow therapeutic index of these agents. Such investigations have led to Clinical Pharmacology FDA Subcommittee recommendations for changes to drug package instructions. This Review discusses the current limitations of body-surface-area-based dosing, examples of successful pharmacogenomic investigations that have used drug-metabolizing enzymes to decrease drug toxicity and/or improve efficacy, and the future promises of pharmacogenomic-directed pharmacotherapy.
Key Points
-
The majority of chemotherapeutic agents are dosed according to the patient's body surface area, which correlates poorly with drug pharmacokinetics
-
Genetic differences in drug-metabolizing enzymes partially explain the interindividual pharmacokinetic variability of many anticancer agents
-
Patients with thiopurine S-methyltransferase deficiency require 5–10% of the standard dose of thiopurine immunosuppressive agents to avoid severe hematologic toxicity
-
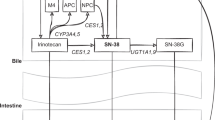
The UGT1A1*28 allele is associated with increased neutropenia and diarrhea in patients receiving irinotecan, especially at doses greater than 200 mg/m2; the FDA has recommended empirical dose reduction in patients who are homozygous for this allele
-
Patients receiving tamoxifen who are classified as CYP2D6 poor metabolizers, on the basis of either genotype or concurrent drug therapy, are likely to have a shorter time to relapse and lower disease-free survival than extensive metabolizers or patients who are homozygous for the wild-type CYP2D6 allele
-
An algorithm that uses patient's height, age and both VKORC1 and CYP2C9 genotypes has demonstrated the ability to account for 54.2% of the variability in warfarin dose requirements and could potentially improve the quality of care provided for patients receiving this anticoagulant
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Baker RJ et al. (1957) The use of surface area for establishing normal blood volume. Surg Gynecol Obstet 104: 183–189
Gurney H (1966) Dose calculation of anticancer drugs: a review of the current practice and introduction of an alternative. J Clin Oncol 14: 2590–2611
Freireich EJ et al. (1966) Quantitative comparison of toxicity of anticancer agents in mouse, rat, hamster, dog, monkey, and man. Cancer Chemother Rep 50: 219–231
Goldsmith MA et al. (1975) Quantitative prediction of drug toxicity in humans from toxicity in small and large animals. Cancer Res 35: 1354–1364
Evans WE and Relling MV (1999) Pharmacogenomics: translating functional genomics into rational therapeutics. Science 286: 487–491
Tai H-L et al. (1999) Enhanced proteasomal degradation of mutant human thiopurine S-methyltransferase (TPMT) in mammalian cells: mechanism for TPMT protein deficiency inherited by TPMT*2, TPMT*3A, TPMT*3B or TPMT*3C. Pharmacogenetics 9: 641–650
Tai H-L et al. (1996) Thiopurine S-methyltransferase deficiency: two nucleotide transitions define the most prevalent mutant allele associated with loss of catalytic activity in Caucasians. Am J Hum Genet 58: 694–702
Tai HL et al. (1997) Enhanced proteolysis of thiopurine S-methyltransferase (TPMT) encoded by mutant alleles in humans (TPMT*3A, TPMT*2): Mechanisms for the genetic polymorphism of TPMT activity. Proc Natl Acad Sci USA 94: 6444–6449
Weinshilboum RM and Sladek SL (1980) Mercaptopurine pharmacogenetics: monogenic inheritance of erythrocyte thiopurine methyltransferase activity. Am J Hum Genet 32: 651–662
McLeod HL et al. (1995) Polymorphic thiopurine methyltransferase in erythrocytes is indicative of activity in leukemic blasts from children with acute lymphoblastic leukemia. Blood 85: 1897–1902
Evans WE et al. (2001) Preponderance of thiopurine S-methyltransferase deficiency and heterozygosity among patients intolerant to mercaptopurine or azathioprine. J Clin Oncol 19: 2293–2301
Evans WE et al. (1991) Altered mercaptopurine metabolism, toxic effects, and dosage requirement in a thiopurine methyltransferase-deficient child with acute lymphocytic leukemia. J Pediatr 119: 985–989
Relling MV et al. (1999) Mercaptopurine therapy intolerance and heterozygosity at the thiopurine S-methyltransferase gene locus. J Natl Cancer Inst 91: 2001–2008
Kelly H and Goldberg RM (2005) Systemic therapy for metastatic colorectal cancer: current options, current evidence. J Clin Oncol 23: 4553–4560
Saltz LB et al. (2000) Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. Irinotecan Study Group. N Engl J Med 343: 905–914
Douillard JY et al. (2000) Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomized trial. Lancet 355: 1041–1047
Vanhoefer U et al. (2001) Irinotecan in the treatment of colorectal cancer: clinical overview. J Clin Oncol 19: 1501–1518
Rothenberg ML et al. (2001) Mortality associated with irinotecan plus bolus fluorouracil/leucovorin: summary findings of an independent panel. J Clin Oncol 19: 3801–3807
Slatter JC et al. (2000) Pharmacokinetics, metabolism, and excretion of irinotecan (CPT-11) following IV infusion of [(14)C]CPT-11 in cancer patients. Drug Metab Dispos 28: 423–433
Iyer L et al. (1998) Genetic predisposition to the metabolism of irinotecan (CPT-11). Role of uridine diphosphate glucuronosyltransferase isoform 1A1 in the glucuronidation of its active metabolite (SN-38) in human liver microsomes. J Clin Invest 101: 847–854
Bosma PJ et al. (1995) The genetic basis of the reduced expression of UDP-glucuronosyltransferase 1in Gilbert's syndrome. N Engl J Med 333: 1171–1175
Monaghan G et al. (1996) Genetic variation in bilirubin UDP-glucuronosyltransferase gene promoter and Gilbert's syndrome. Lancet 347: 578–581
Hall D et al. (1999) Variability at the uridine diphosphate glucuronosyltransferase 1A1 promoter in human populations and primates. Pharmacogenetics 9: 591–599
Wasserman E et al. (1997) Severe CPT-11 toxicity in patients with Gilbert's syndrome: two case reports. Ann Oncol 8: 1049–1051
Innocenti F et al. (2004) Genetic variants in the UDP-glucuronosyltransferase 1A1 gene predict the risk of severe neutropenia of irinotecan. J Clin Oncol 22: 1382–1388
Iyer L et al. (2002) UGT1A1*28 polymorphism as a determinant of irinotecan disposition and toxicity. Pharmacogenomics. J 2: 43–47
Ando Y et al. (2000) Polymorphisms of UDP-glucuronosyltransferase gene and irinotecan toxicity: a pharmacogenetic analysis. Cancer Res 60: 6921–6926
Hoskins JM et al. (2007) UGT1A1*28 genotype and irinotecan-induced neutropenia: dose matters. J Natl Cancer Inst 99: 1290–1295
Osborne CK (1998) Tamoxifen in the treatment of breast cancer. N Engl J Med 339: 1609–1618
Dellapasqua S et al. (2005) Adjuvant endocrine therapy for premenopausal women with early breast cancer. J Clin Oncol 23: 1736–1750
Early Breast Cancer Trialists' Collaborative Group (1998): Tamoxifen for early breast cancer: overview of the randomized trials. Lancet 351: 1451–1467
Jordan VC et al. (1977) A monohydroxylated metabolite of tamoxifen with potent antiestrogenic activity. J Endocrinol 75: 305–316
Stearns V et al. (2003) Active tamoxifen metabolite plasma concentrations after coadministration of tamoxifen and the selective serotonin reuptake inhibitor paroxetine. J Natl Cancer Inst 95: 1758–1764
Lee KH et al. (2003) Quantification of tamoxifen and three metabolites in plasma by high-performance liquid chromatography with fluorescence detection: application to a clinical trial. J Chromatogr B Analyt Technol Biomed Life Sci 791: 245–253
Desta Z et al. (2004) Comprehensive evaluation of tamoxifen sequential biotransformation by the human cytochrome P450 system in vitro: prominent roles for CYP3A and CYP2D6. J Pharmacol Exp Ther 310: 1062–1075
Bradford LD (2002) CYP2D6 allele frequency in European Caucasians, Asians, Africans and their descendents. Pharmacogenomics 3: 229–243
Jin Y et al. (2005) CYP2D6 genotype, antidepressant use, and tamoxifen metabolism during adjuvant breast cancer treatment. J Natl Cancer Inst 97: 30–39
Goetz MP et al. (2005) Pharmacogenetics of tamoxifen biotransformation is associated with clinical outcomes of efficacy and hot flashes. J Clin Oncol 23: 9312–9318
Hyeong-Seok L et al. (2007) Clinical implications of CYP2D6 genotypes predictive of tamoxifen pharmacokinetics in metastatic breast cancer patients. J Clin Oncol 25: 3837–3845
Goetz MP et al. (2007) The impact of cytochrome P450 2D6 metabolism in women receiving adjuvant tamoxifen. Breast Cancer Res Treat 101: 113–121
Borges S et al. (2006) Quantitative effect of CYP2D6 genotype and inhibitors on tamoxifen metabolism: implication for optimization of breast cancer treatment. Clin Pharmcol Ther 80: 61–74
Schroth W et al. (2007) Breast cancer treatment outcome with adjuvant tamoxifen relative to patient CYP2D6 and CYP2C19 genotypes. J Clin Oncol 25: 5187–5193
Ansell J et al. (2004) The pharmacolology and management of the vitamin K antagonists. Chest 126 (Suppl): S204–S233
Fihn SD et al. (1993) Risk factors for complications of chronic anticoagulation. Ann Intern Med 118: 511–520
Breckenridge A et al. (1974) Pharmacokinetics and pharmacodynamics of the enantiomers of warfarin in man. Clin Pharmacol Ther 15: 424–430
Takahashi H and Echizen H (2001) Pharmacogenetics of warfarin elimination and its clinical implications. Clin Pharmacokinet 40: 587–603
Takanaski K et al. (2000) CYP2C9 Ile359 and Leu359 variants. Pharmacogenetics 10: 95–104
Scordo MG et al. (2001) Genetic polymorphism of cytochrome P450 2C9 in a Caucasian and a black African population. Br J Pharmacol 52: 447–450
Choonara IA et al. (1988) The relationship between inhibition of vitamin K1 2,3 epoxide reductase and reduction of clotting factor activity with warfarin. Br J Clin Pharmacol 25: 1–7
Higashi MK et al. (2002) Association between CYP2C9 genetic variants and anticoagulation-related outcomes during warfarin therapy. JAMA 287: 1690–1698
Sconce EA et al. (2005) The impact of CYP2C9 and VKORC1 genetic polymorphisms and patient characteristics upon warfarin dose requirements: proposal for a new dosing regimen. Blood 106: 2329–2333
Rost S et al. (2004) Mutations in VKORC1 cause warfarin resistance and multiple coagulation factor deficiency type 2. Nature 427: 537–541
Crespi CL and Miller VP (1997) The R144C change in the CYP2C9*2 allele alters interaction of the cytochrome P450 with NADPH:cytochrome P450 oxidoreductase. Pharmacogenetics 7: 203–210
Rieder MJ et al. (2005) Effect of VKORC1 haplotypes on transcriptional regulation and warfarin dose. N Engl J Med 352: 2285–2293
Warfarin Dosing [http://www.warfarindosing.org]
Mattison LK et al. (2006) The uracil breath test in the assessment of dihydropyrimidine dehydrogenase activity: pharmacokinetic relationship between expired 13CO2 and plasma [2-13C]dihydrouracil. Clin Cancer. Res 12: 549–555
Marcuello E et al. (2004) UGT1A1 gene variations and irinotecan treatment in patients with metastatic colorectal cancer. Br J Cancer 91: 678–682
Rouits E et al. (2004) Relevance of different UGT1A1 polymorphisms in irinotecan-induced toxicity: a molecular and clinical study of 75 patients. Clin Cancer Res 10: 5151–5159
Toffoli G et al. (2006) The role of UGT1A1*28 polymorphism in the pharmacodynamics and pharmacokinetics of irinotecan in patients with metastatic colorectal cancer. J Clin Oncol 24: 3061–3068
Pillot GA et al. (2006) A phase II study of irinotecan and carboplatin in advanced non-small cell lung cancer with pharmacogenomics analysis: final report. J Thorac Oncol 1: 972–978
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Dr Howard McLeod declared he is a consultant for Affymetrix and Myriad Genetics. CM Walko declared no competing interests.
Rights and permissions
About this article
Cite this article
Walko, C., McLeod, H. Pharmacogenomic progress in individualized dosing of key drugs for cancer patients. Nat Rev Clin Oncol 6, 153–162 (2009). https://doi.org/10.1038/ncponc1303
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncponc1303
This article is cited by
-
Personalized Genomic Results: Analysis of Informational Needs
Journal of Genetic Counseling (2014)
-
Envisioning the future of early anticancer drug development
Nature Reviews Cancer (2010)