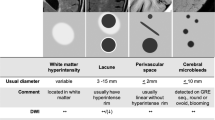
Abstract
The extent to which white matter changes affect brain function in elderly individuals is a matter for debate. Although there is a consensus that large confluent white matter lesions (WMLs) can be attributed to small-vessel disease and might denote anatomical damage to axons, the clinical effect of WMLs with regard to cognitive impairment is less certain. In this Review, we argue that WMLs are associated with greater detectable progressive cognitive deterioration than is normal aging, but other causes of progressive cognitive deterioration, such as Alzheimer's disease, are associated with greater cognitive decline than are WMLs. This view has important implications for the development of drugs for the treatment and prevention of cognitive impairment and dementia.
Key Points
-
Focal white matter lesions (WMLs) can be attributed to small-vessel disease and are associated with variable degrees of demyelination, axonal loss and gliosis
-
The effect of WMLs on global cognitive performance in cross-sectional studies is relatively small, even for severe lesions, and amounts at most to 0.7 points out of a maximum of 30 on the Mini-Mental State Examination scale
-
In longitudinal studies, the rate of cognitive deterioration attributable to WMLs is, on average, 12 times lower than that attributable to Alzheimer's disease
-
Only individuals with the more-severe degrees of WMLs have clinically relevant cognitive decline, and these patients might be candidates for drug therapy targeted to small-vessel cerebrovascular disease
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Hachinski VC et al. (1987) Leuko-araiosis. Arch Neurol 44: 21–23
Gootjes L et al. (2004) Regional distribution of white matter hyperintensities in vascular dementia, Alzheimer's disease and healthy aging. Dement Geriatr Cogn Disord 18: 180–188
Fazekas F et al. (1993) Pathologic correlates of incidental MRI white matter signal hyperintensities. Neurology 43: 1683–1689
Pantoni L and Garcia JH (1997) Pathogenesis of leukoaraiosis: a review. Stroke 28: 652–659
Fazekas F et al. (1998) Pathophysiologic mechanisms in the development of age-related white matter changes of the brain. Dement Geriatr Cogn Disord 9 (Suppl 1): S2–S5
Burns JM et al. (2005) White matter lesions are prevalent but differentially related with cognition in aging and early Alzheimer disease. Arch Neurol 62: 1870–1876
van der Flier W et al. (2005) Small vessel disease and general cognitive function in nondisabled elderly: the LADIS study. Stroke 36: 2116–2120
Prins ND et al. (2004) Cerebral white matter lesions and the risk of dementia. Arch Neurol 61: 1531–1534
Prins ND et al. (2005) Cerebral small-vessel disease and decline in information processing speed, executive function and memory. Brain 128: 2034–2041
Jones DK et al. (1999) Characterization of white matter damage in ischemic leukoaraiosis with diffusion tensor MRI. Stroke 30: 393–407
Nave RD et al. (2007) Whole-brain histogram and voxel-based analyses of diffusion tensor imaging in patients with leukoaraiosis: correlation with motor and cognitive impairment. AJNR Am J Neuroradiol 28: 1313–1319
Charlton RA et al. (2006) White matter damage on diffusion tensor imaging correlates with age-related cognitive decline. Neurology 24: 217–222
Nitkunan A et al. (2006) Correlations between MRS and DTI in cerebral small vessel disease. NMR Biomed 19: 610–616
Medina D et al. (2006) White matter changes in mild cognitive impairment and AD: a diffusion tensor imaging study. Neurobiol Aging 27: 663–672
Bartzokis G et al. (2003) White matter structural integrity in healthy aging adults and patients with Alzheimer disease: a magnetic resonance imaging study. Arch Neurol 60: 393–398
Brooks WM et al. (1997) 1H-MRS differentiates white matter hyperintensities in subcortical arteriosclerotic encephalopathy from those in normal elderly. Stroke 28: 1940–1943
O'Sullivan M et al. (2004) Diffusion tensor MRI correlates with executive dysfunction in patients with ischaemic leukoaraiosis. J Neurol Neurosurg Psychiatry 75: 441–447
O'Sullivan M et al. (2001) Normal-appearing white matter in ischemic leukoaraiosis: a diffusion tensor MRI study. Neurology 57: 2307–2310
Fazekas F et al. (2005) MTI of white matter hyperintensities. Brain 128: 2926–2932
Deary IJ et al. (2006) White matter integrity and cognition in childhood and old age. Neurology 66: 505–512
Dufouil C et al. (2003) Influence of education on the relationship between white matter lesions and cognition. Neurology 60: 831–836
Longstreth WT Jr et al. (1996) Clinical correlates of white matter findings on cranial magnetic resonance imaging of 3301 elderly people: the Cardiovascular Health Study. Stroke 27: 1274–1282
de Groot JC et al. (2000) Cerebral white matter lesions and cognitive function: the Rotterdam Scan Study. Ann Neurol 47: 145–151
Gouw AA et al. (2006) Simple versus complex assessment of white matter hyperintensities in relation to physical performance and cognition: the LADIS study. J Neurol 253: 1189–1196
Au R et al. (2006) Association of white matter hyperintensity volume with decreased cognitive functioning: the Framingham Heart Study. Arch Neurol 63: 246–250
Soderlund H et al. (2006) Cerebral changes on MRI and cognitive function: the CASCADE study. Neurobiol Aging 27: 16–23
Gunning-Dixon FM and Raz N (2000) The cognitive correlates of white matter abnormalities in normal aging: a quantitative review. Neuropsychology 14: 224–232
Agüero-Torres H et al. (2002) The impact of somatic and cognitive disorders on the functional status of the elderly. J Clin Epidemiol 55: 1007–1012
Comijs HC et al. (2005) The impact of change in cognitive functioning and cognitive decline on disability, well-being, and the use of healthcare services in older persons: results of Longitudinal Aging Study Amsterdam. Dement Geriatr Cogn Disord 19: 316–323
Cahn-Weiner DA et al. (2002) Tests of executive function predict instrumental activities of daily living in community-dwelling older individuals. Appl Neuropsychol 9: 187–191
Rapp MA et al. (2005) Relationship of neuropsychological performance to functional status in nursing home residents and community-dwelling older adults. Am J Geriatr Psychiatry 13: 450–459
Burback D et al. (1999) Key methodological features of randomized controlled trials of Alzheimer's disease therapy: minimal clinically important difference, sample size and trial duration. Dement Geriatr Cogn Disord 10: 534–540
Hachinski V et al. (2006) National Institute of Neurological Disorders and Stroke–Canadian Stroke Network vascular cognitive impairment harmonization standards. Stroke 37: 2220–2241
O'Sullivan M et al. (2005) Brief cognitive assessment for patients with cerebral small vessel disease. J Neurol Neurosurg Psychiatry 76: 1140–1145
Bennett DA et al. (2006) Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology 66: 1837–1844
Nolan KA et al. (1998) Absence of vascular dementia in an autopsy series from a dementia clinic. J Am Geriatr Soc 46: 597–604
Winnock M et al. (2002) Longitudinal analysis of the effect of apolipoprotein E ε4 and education on cognitive performance in elderly subjects: the PAQUID study. J Neurol Neurosurg Psychiatry 72: 794–797
Clark CM et al. (1999) Variability in annual Mini-Mental State Examination score in patients with probable Alzheimer disease: a clinical perspective of data from the Consortium to Establish a Registry for Alzheimer's Disease. Arch Neurol 56: 857–862
Ross ED et al. (2005) Relationship of leukoaraiosis to cognitive decline and cognitive aging. Cogn Behav Neurol 18: 89–97
Breteler MM et al. (1994) Cerebral white matter lesions, vascular risk factors, and cognitive function in a population-based study: the Rotterdam Study. Neurology 44: 1246–1252
De Groot JC et al. (2002) Periventricular cerebral white matter lesions predict rate of cognitive decline. Ann Neurol 52: 335–341
Bowler JV and Gorelick PB (2007) Advances in vascular cognitive impairment 2006. Stroke 38: 241–244
Snowdon DA et al. (1997) Brain infarction and the clinical expression of Alzheimer disease: the Nun Study. JAMA 277: 813–817
Smith CD et al. (2000) White matter volumes and periventricular white matter hyperintensities in aging and dementia. Neurology 54: 838–842
Starr JM et al. (1997) Age-associated cognitive decline in healthy old people. Age Ageing 26: 295–300
Lobo A et al. (2000) Prevalence of dementia and major subtypes in Europe: a collaborative study of population-based cohorts. Neurologic Diseases in the Elderly Research Group. Neurology 54 (Suppl 5): S4–S9
Longstreth WT Jr et al. (2005) Incidence, manifestations, and predictors of worsening white matter on serial cranial magnetic resonance imaging in the elderly: the Cardiovascular Health Study. Stroke 36: 56–61
Jellinger KA (2002) Alzheimer disease and cerebrovascular pathology: an update. J Neural Transm 109: 813–836
Inzitari D et al. (2007) Risk of rapid global functional decline in elderly patients with severe cerebral age-related white matter changes: the LADIS study. Arch Intern Med 167: 81–88
Schmidt R et al. (2007) Progression of leukoaraiosis and cognition. Stroke 38: 2619–2625
Roberson ED and Mucke L (2006) 100 years and counting: prospects for defeating Alzheimer's disease. Science 314: 781–784
Dufouil C et al. (2005) Effects of blood pressure lowering on cerebral white matter hyperintensities in patients with stroke: the PROGRESS (Perindopril Protection Against Recurrent Stroke Study) magnetic resonance imaging substudy. Circulation 112: 1644–1650
Fazekas F et al. (1987) MR signal abnormalities at 1.5 T in Alzheimer's dementia and normal aging. AJR Am J Roentgenol 149: 351–356
Jim version 3.0 (Xinapse Systems, Thorpe Waterville, UK)
Pantoni L et al. (2002) Visual rating scales for age-related white matter changes (leukoaraiosis): can the heterogeneity be reduced? Stroke 33: 2827–2833
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Frisoni, G., Galluzzi, S., Pantoni, L. et al. The effect of white matter lesions on cognition in the elderly—small but detectable. Nat Rev Neurol 3, 620–627 (2007). https://doi.org/10.1038/ncpneuro0638
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ncpneuro0638
This article is cited by
-
Cerebral Superficial Siderosis
Clinical Neuroradiology (2023)
-
Increased risk of cerebral white matter lesions in obstructive sleep apnea syndrome
Sleep and Biological Rhythms (2017)
-
Cerebrovascular Dysfunction in Preeclamptic Pregnancies
Current Hypertension Reports (2015)
-
Altered human brain anatomy in chronic smokers: a review of magnetic resonance imaging studies
Neurological Sciences (2015)
-
Dietary glycaemic load associated with cognitive performance in elderly subjects
European Journal of Nutrition (2015)