Abstract
Statins, a family of 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors, are used primarily to reduce atherogenesis and cardiovascular morbidity. Surprisingly, they have also been shown to have immunomodulatory properties that might be of benefit for the treatment of autoimmune disorders. Statins can prevent and even reverse ongoing paralysis in experimental autoimmune encephalomyelitis—the mouse model for multiple sclerosis—and on the basis of these findings, statins are now being tested in patients with multiple sclerosis in clinical trials.
Key Points
-
Statins are cholesterol-lowering drugs with immunomodulatory properties that might be of benefit in the treatment of neuroinflammatory disorders such as multiple sclerosis (MS)
-
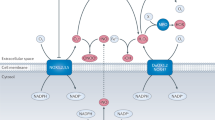
Most of the currently known statin-mediated immunomodulatory effects seem to be related to the inhibition of HMG-CoA reductase, a key enzyme in the cholesterol biosynthetic pathway
-
Studies in mice with experimental autoimmune encephalomyelitis have shown that statins interfere with multiple aspects of immune cell function
-
At present, there is insufficient epidemiological evidence to support the use of statins to treat MS in humans, but the initial results of clinical trials have been encouraging
-
Statins are also being tested in other inflammatory conditions, including rheumatoid arthritis and Alzheimer's disease
-
Although statins are perceived as safe and well-tolerated drugs, they have side effects that should be considered, particularly when they are administered in combination with other agents
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Ginsberg HN (1998) Effects of statins on triglyceride metabolism. Am J Cardiol 81: 32B–35B
Kobashigawa JA et al. (1995) Effect of pravastatin on outcomes after cardiac transplantation. N Engl J Med 333: 621–627
Steinman L (2004) Immune therapy for autoimmune diseases. Science 305: 212–216
Weitz-Schmidt G et al. (2001) Statins selectively inhibit leukocyte function antigen-1 by binding to a novel regulatory integrin site. Nat Med 7: 687–692
Youssef S et al. (2002) The HMG-CoA reductase inhibitor, atorvastatin, promotes a Th2 bias and reverses paralysis in central nervous system autoimmune disease. Nature 420: 78–84
Aktas O et al. (2003) Treatment of relapsing paralysis in experimental encephalomyelitis by targeting Th1 cells through atorvastatin. J Exp Med 197: 725–733
Stanislaus R et al. (2002) Immunomodulation of experimental autoimmune encephalomyelitis in the Lewis rats by lovastatin. Neurosci Lett 333: 167–170
Chang CH and Flavell RA (1995) Class II transactivator regulates the expression of multiple genes involved in antigen presentation. J Exp Med 181: 765–767
Kwak B et al. (2000) Statins as a newly recognized type of immunomodulator. Nat Med 6: 1399–1402
Dustin ML and Shaw AS (1999) Costimulation: building an immunological synapse. Science 283: 649–650
Stüve O et al. (2004) Atorvastatin enhances the clinically beneficial effect of Glatiramer Acetate in Experimental Autoimmune Encephalomyelitis through Induction of a Th2 Phenotype [abstract #S50.003]. In Proceedings of the 56th Meeting of the American Academy of Neurology: 2004 April 24–May 1; San Francisco. St Paul: American Academy of Neurology Press
Abbas AK et al. (1996) Functional diversity of helper T lymphocytes. Nature 383: 787–793
Panitch HS et al. (1987) Exacerbations of multiple sclerosis in patients treated with gamma interferon. Lancet 1: 893–895
Khoury SJ et al. (1992) Oral tolerance to myelin basic protein and natural recovery from experimental autoimmune encephalomyelitis are associated with downregulation of inflammatory cytokines and differential upregulation of transforming growth factor beta, interleukin 4, and prostaglandin E expression in the brain. J Exp Med 176: 1355–1364
Begolka WS et al. (1998) Differential expression of inflammatory cytokines parallels progression of central nervous system pathology in two clinically distinct models of multiple sclerosis. J Immunol 161: 4437–4446
Neuhaus O et al. (2002) Statins as immunomodulators: comparison with interferon-beta 1b in MS. Neurology 59: 990–997
Nath N et al. (2004) Potential targets of 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor for multiple sclerosis therapy. J Immunol 172: 1273–1286
Hakamada-Taguchi R et al. (2003) Inhibition of hydroxymethylglutaryl-coenzyme A reductase reduces Th1 development and promotes Th2 development. Circ Res 93: 948–956
Waiczies S et al. (2005) Atorvastatin induces T cell anergy via phosphorylation of ERK1. J Immunol 174: 5630–5635
Dunn SE et al. (2005) Atorvastatin prevents the Th1 differentiation of myelin-reactive T cells during CNS autoimmune disease by interfering with the prenylation of Ras and activation of ERK pathway [abstract #S64.001]. In Proceedings of the 57th Meeting of the American Academy of Neurology: 2005 April 9–16; Miami Beach. St Paul: American Academy of Neurology Press
Jorritsma et al. (2003) Role of TCR-induced extracellular signal-regulated kinase activation in the regulation of early IL-4 expression in naive CD4+ T cells. J Immunol 170: 2427–2434
Cannella B and Raine CS (1995) The adhesion molecule and cytokine profile of multiple sclerosis lesions. Ann Neurol 37: 424–435
Etienne S et al. (1998) ICAM-1 signaling pathways associated with Rho activation in microvascular brain endothelial cells. J Immunol 161: 5755–5761
Adamson P et al. (1999) Lymphocyte migration through brain endothelial cell monolayers involves signaling through endothelial ICAM-1 via a rho-dependent pathway. J Immunol 162: 2964–2973
Etienne-Manneville S et al. (2000) ICAM-1-coupled cytoskeletal rearrangements and transendothelial lymphocyte migration involve intracellular calcium signaling in brain endothelial cell lines. J Immunol 165: 3375–3383
Adamson P et al. (2002) Lymphocyte trafficking through the blood–brain barrier is dependent on endothelial cell heterotrimeric G-protein signaling. Faseb J 16: 1185–1194
Walters CE et al. (2002) Inhibition of Rho GTPases with protein prenyltransferase inhibitors prevents leukocyte recruitment to the central nervous system and attenuates clinical signs of disease in an animal model of multiple sclerosis. J Immunol 168: 4087–4094
Greenwood J et al. (2003) Lovastatin inhibits brain endothelial cell Rho-mediated lymphocyte migration and attenuates experimental autoimmune encephalomyelitis. Faseb J 17: 905–907
Yong VW et al. (1998) Matrix metalloproteinases and diseases of the CNS. Trends Neurosci 21: 75–80
Ganne F et al. (2000) Cerivastatin, an inhibitor of HMG-CoA reductase, inhibits urokinase/urokinase-receptor expression and MMP-9 secretion by peripheral blood monocytes—a possible protective mechanism against atherothrombosis. Thromb Haemost 84: 680–688
Bellosta S et al. (1998) HMG-CoA reductase inhibitors reduce MMP-9 secretion by macrophages. Arterioscler Thromb Vasc Biol 18: 1671–1678
Bonet S et al. (1999) When and how do we treat our hypercholesterolemic patients? Aten Primaria 24: 397–403
Sena A et al. (2003) Therapeutic potential of lovastatin in multiple sclerosis. J Neurol 250: 754–755
Vollmer T et al. (2004) Oral simvastatin treatment in relapsing-remitting multiple sclerosis. Lancet 363: 1607–1608
Birnbaum G and Altafullah I (2005) A double blind, placebo controlled combination trial of interferon beta 1a (Rebif) and atorvastatin (Lipitor) in patients with relapsing remitting multiple sclerosis [abstract #P06.158]. In Proceedings of the 57th Meeting of the American Academy of Neurology: 2005 April 9–16; Miami Beach. St Paul: American Academy of Neurology Press
Neuhaus O et al. (2001) Mechanisms of action of glatiramer acetate in multiple sclerosis. Neurology 56: 702–708
Duda PW et al. (2000) Glatiramer acetate (Copaxone) induces degenerate, Th2-polarized immune responses in patients with multiple sclerosis. J Clin Invest 105: 967–976
Kim HJ et al. (2004) Type 2 monocyte and microglia differentiation mediated by glatiramer acetate therapy in patients with multiple sclerosis. J Immunol 172: 7144–7153
Weber MS et al. (2004) Multiple sclerosis: glatiramer acetate inhibits monocyte reactivity in vitro and in vivo. Brain 127: 1370–1378
McCarey DW et al. (2004) Trial of Atorvastatin in Rheumatoid Arthritis (TARA): double-blind, randomised placebo-controlled trial. Lancet 363: 2015–2021
Wolozin B et al. (2000) Decreased prevalence of Alzheimer disease associated with 3-hydroxy-3-methyglutaryl coenzyme A reductase inhibitors. Arch Neurol 57: 1439–1443
Jick H et al. (2000) Statins and the risk of dementia. Lancet 356: 1627–1631
Sparks DL et al. (2005) Atorvastatin for the treatment of mild to moderate Alzheimer disease: preliminary results. Arch Neurol 62: 753–757
Sparks DL et al. (1994) Induction of Alzheimer-like beta-amyloid immunoreactivity in the brains of rabbits with dietary cholesterol. Exp Neurol 126: 88–94
Refolo LM et al. (2000) Hypercholesterolemia accelerates the Alzheimer's amyloid pathology in a transgenic mouse model. Neurobiol Dis 7: 321–331
Fassbender K et al. (2001) Simvastatin strongly reduces levels of Alzheimer's disease beta-amyloid peptides Aβ42 and Aβ40 in vitro and in vivo. Proc Natl Acad Sci USA 98: 5856–5861
Akiyama H et al. (2000) Inflammation and Alzheimer's disease. Neurobiol Aging 21: 383–421
[No authors listed] (1994) Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 344: 1383–1389
Jukema JW et al. (1995) Effects of lipid lowering by pravastatin on progression and regression of coronary artery disease in symptomatic men with normal to moderately elevated serum cholesterol levels: The Regression Growth Evaluation Statin Study (REGRESS). Circulation 91: 2528–2540
Graham DJ et al. (2004) Incidence of hospitalized rhabdomyolysis in patients treated with lipid-lowering drugs. JAMA 292: 2585–2590
Staffa JA et al. (2002) Cerivastatin and reports of fatal rhabdomyolysis. N Engl J Med 346: 539–540
Gaist D et al. (2002) Statins and risk of polyneuropathy: a case-control study. Neurology 58: 1333–1337
Gaist D et al. (2001) Are users of lipid-lowering drugs at increased risk of peripheral neuropathy? Eur J Clin Pharmacol 56: 931–933.
Pahan K et al. (1997) Lovastatin and phenylacetate inhibit the induction of nitric oxide synthase and cytokines in rat primary astrocytes, microglia, and macrophages. J Clin Invest 100: 2671–2679
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Weber, M., Prod'homme, T., Steinman, L. et al. Drug Insight: using statins to treat neuroinflammatory disease. Nat Rev Neurol 1, 106–112 (2005). https://doi.org/10.1038/ncpneuro0047
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ncpneuro0047
This article is cited by
-
The Use of Oral Disease-Modifying Therapies in Multiple Sclerosis
Current Neurology and Neuroscience Reports (2016)
-
Neither T-helper type 2 nor Foxp3+regulatory T cells are necessary for therapeutic benefit of atorvastatin in treatment of central nervous system autoimmunity
Journal of Neuroinflammation (2014)
-
Multiple sclerosis therapy: An update on recently finished trials
Journal of Neurology (2007)