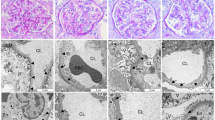
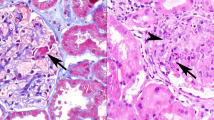
Abstract
Viral infections can cause many glomerular diseases. The diagnostic criteria for virus-related nephropathy include detailed clinical and laboratory data, and tissue molecular analysis. Several mechanisms are involved in the pathogenesis of virus-related nephropathy, including tropism of the virus in the kidney, induction of abnormal immune complexes, direct cytopathogenic effects, and multiorgan failure. Hepatitis B virus is associated with membranous nephropathy and mesangiocapillary glomerulonephritis in endemic areas. Hepatitis C virus causes various forms of glomerulonephritis, including cryoglobulinemia-mediated glomerulonephritis. Infection with HIV is associated with a collapsing focal segmental glomerulosclerosis, a distinctive disease that affects mainly Africans and African Americans. In the course of HIV infection, other types of immune complex glomerulonephritis can occur, most frequently in whites. Recent reports indicate a role for parvovirus B19 in 'idiopathic' collapsing focal segmental glomerulosclerosis. Both hantaviruses, and coronaviruses associated with severe acute respiratory syndrome, can lead to acute renal failure. Renal biopsy followed by appropriate serological and molecular testing is essential for defining virus-related glomerular lesions and guiding prognostic and therapeutic evaluation.
Key Points
-
Different viruses use different strategies to induce viral nephropathy
-
Some of the pathogenic mechanisms and molecules underlying viral nephropathy are direct cytopathogenic effects on glomerular and tubulointerstitial cells, circulating immune complexes, hemodynamic perturbation and rhabdomyolysis
-
Different viruses cause different forms of nephropathy
-
Hepatitis A, parvovirus B19 and Epstein–Barr virus cause acute glomerulonephritis; hepatitis B and C, HIV and parvovirus B19 cause chronic glomerulonephritis; hantavirus, severe acute respiratory syndrome coranavirus, BK virus and influenza A cause interstitial nephritis
Similar content being viewed by others
Introduction
The pathogenetic links between viral infection and renal disease are often difficult to establish. The criteria for proving causality are complex and include (besides recognition of the clinical syndrome) serological diagnosis, identification of specific viral antigenemia, and detection in glomerular structures of viral antigens and host antibodies. According to Koch's postulates, the etiological link should be confirmed by complete cure following eradication of the virus, but this is not always possible. The concentration of viral antigens is higher in tissue than in the circulation, where antigens are complexed with specific autoantibodies. Virological and molecular analysis of pathologic tissues by in situ hybridization, polymerase chain reaction and ultrastructural analysis led to successful detection and identification of the virus. It should be noted that tubular uptake of viral particles is common and does not necessarily establish an etiological link with renal disease. Improvement of the renal disease concomitant with clearance of the suspected antigen, or recurrence of glomerulonephritis following reinfection, are additional clinical criteria.
Viruses and mechanisms involved in viral nephropathy
Different mechanisms are operative in different viral nephropathies (Box 1). In acute glomerulonephritis, direct viral infection of the glomerulus induces proliferative changes following release of cytokines.1 The nephropathy is reversible in most cases if the virus is rapidly cleared. In chronic forms of glomerulonephritis, persistent viral infection provides continuous antigenic stimulation, resulting in antibody production and formation of immune complexes. Studies indicate a role in the disease pathogenesis for these immune complexes, which can be derived from the circulation or formed in situ.2,3 Viral proteins cause inflammatory renal diseases via synthesis of various mediators that can cause sclerosis and worsen glomerulopathy.4,5 A direct cytopathic effect of viral proteins has also been postulated.6 In hepatitis C virus (HCV)-induced mesangiocapillary glomerulonephritis (MCGN), production of circulating cryoglobulins is induced as an abnormal host response to infection. Cryoglobulins are either type II or type III. At least two classes of immunoglobulins are involved, one of which is polyclonal.7 In acute renal failure associated with infection by hantavirus or severe acute respiratory syndrome coronavirus, the pathogenetic mechanisms of interstitial nephritis, disseminated intravascular coagulopathy, and multiorgan failure—rather than formation of immune complexes—are predominant.
Box 2 lists the viruses that are known to induce renal diseases. Globally, the most frequent and well recognized virus-related glomerulonephropathies are those associated with hepatitis B virus (HBV), in which formation of immune complexes is important. HCV is the etiological agent of cryoglobulinemia-related MCGN in most cases. Infection with HIV can induce a broad spectrum of glomerular lesions via multiple pathogenic mechanisms. Parvovirus B19 (PVB19) is associated with non-HIV collapsing glomerulopathy,8 idiopathic focal segmental glomerulosclerosis (FSGS),9 and immune complex glomerulonephritis.10 Polyoma BK virus and hantavirus most frequently cause tubulointerstitial damage; occasionally, virus is simultaneously localized to the glomerulus. A rare or speculative role in glomerulonephritides is currently attributed to other viruses, such as those causing yellow fever,11 mumps,12 measles,13 varicella,14 and herpes.15
Glomerulonephritis associated with hepatitis B virus
HBV is a hepatotropic, double-stranded DNA virus of the Hepadnaviridae family. HBV itself is not cytopathic; hepatitis develops as a result of the host's immune reaction to infected hepatocytes. HBV uses reverse transcriptase to transcribe RNA into DNA. Unlike retroviruses, however, HBV DNA is not integrated into host cell DNA during replication. After an HBV particle binds to and enters a hepatocyte, HBV DNA enters the cell's nucleus and is converted into covalently closed circular DNA. This highly stable genetic material acts as the intermediate template for transcription of RNA copies. This pregenomic messenger RNA is transported to the cytoplasm. It has dual functions: acting as a template for synthesis of new HBV DNA, and carrying genetic information to direct synthesis of viral proteins.
Today, an estimated 350–400 million people worldwide are infected with HBV. In endemic areas, transmission is usually vertical—that is, from infected mother to child. Horizontal transmission occurs via direct contact with blood (e.g. during blood transfusions) or mucous membranes (e.g. during sexual contact), or via the percutaneous route upon contact with blood or body fluids (e.g. during intravenous drug use and needle sharing). Familial clustering of the virus occurs in some regions.
The reported prevalence of HBV-associated nephropathy closely parallels the geographic patterns of prevalence of HBV.16 The three main forms of glomerulonephritis associated with HBV infection are membranous glomerulonephritis, MCGN and IgA nephropathy (IgAN). Membranous glomerulonephritis is most frequently reported in Asian populations17 and in children,18 particularly male children.16 By contrast, mesangial proliferative forms with IgA deposits seem to be most common in adults.19
Three types of glomerulonephritis with pathologic characteristics similar to the human subtypes have been described in woodchucks chronically infected with hepatitis virus.20 As in humans, the membranous pattern of injury most frequently affects young woodchucks, whereas the mesangial proliferative pattern of injury tends to affect older animals. The male : female ratio of affected woodchucks was significantly greater than that of the chronic carrier population.20
Diagnosis and monitoring
In most reports, diagnosis of HBV-associated glomerulonephritis has been based on persistence of circulating HBV or HBV DNA, absence of other causative agents, and presence of HBV-specific antigen(s) or viral genome in the glomerulus. One major difference between the human and woodchuck studies is that the hepatitis B e antigen (HBeAg) system has not been characterized in the latter. In clinical practice, regression of pathology following viral eradication is not easy to demonstrate because of ethical concerns relating to repeat renal biopsies in humans subsequent to clinical remission. As such, the diagnosis of HBV-associated renal disease usually relies heavily upon detection of HBV-specific antigen(s) in glomeruli.
Laboratory testing for diagnosis and assessment of response to treatment should include standard liver biochemistries (serum alanine aminotransferase, γ-glutamyltransferase, and bilirubin levels), and HBV serologies (hepatitis B surface antigen, HBeAg, anti-hepatitis B e, and anti-hepatitis B core antigen antibodies). HBeAg is present in 80% of patients, who might also have high titers of anti-hepatitis B core antigen.21 Subjects with biochemical hepatitis should be tested for circulating HBV DNA22 and undergo liver biopsy. An α-fetoprotein assay could be an important adjunct.23 Serum C3 and C4 levels can be low in 20–50% of patients.
Clinical characteristics
HBV-related membranous nephropathy tends to manifest slightly differently in pediatric and adult patients. In children, there is a strong male preponderance, and the most frequent presentation is nephrotic syndrome, microscopic hematuria, and normal or mildly impaired renal function.24 Pediatric chronic HBV carriers often do not have overt liver disease, and transaminase levels are usually normal. In adults, proteinuria or the nephrotic syndrome are the most common manifestations. Adult male predominance is less obvious than in pediatric populations. Adults are more likely than children to have hypertension, renal dysfunction, and clinical evidence of liver disease.
The prognosis of HBV-associated membranous nephropathy in children is favorable. Stable renal function and high rates of spontaneous remission have been reported in several geographical areas in which disease prevalence is high. By contrast, adults with HBV-associated membranous nephropathy typically develop progressive disease. In Hong Kong, up to 29% of patients had progressive renal failure, and another 10% developed terminal uremia over 5 years.21 The prognosis is even worse for patients with nephrotic-range proteinuria and abnormal liver function tests at presentation. Over 50% of these patients require renal replacement therapy within 3 years.25 Vertical transmission is associated with poorer outcomes than horizontal transmission, as is endemic versus sporadic infection.21,26
As mentioned above, HBV infection can also cause MCGN (with or without cryoglobulinemia), mesangial proliferative glomerulonephritis, and IgAN.19,27 Polyarteritis nodosa has been reported in some patients with HBV and might respond to treatment with corticosteroids and interferon-α.28 Occasional concomitance of the pathologic subtypes can lead to 'double' glomerulopathies. For instance, membranous nephropathy and IgAN have been reported to coexist in an HBV carrier.29
Treatment
Unlike affected children, who have a high rate of spontaneous remission,30 adults with HBV-associated membranous nephropathy typically develop progressive disease.21 Various management strategies have been tried, but an ideal agent is yet to be found. Treatment for HBV-associated renal disease should ideally achieve the following objectives: (i) amelioration of nephrotic syndrome and its complications; (ii) preservation of renal function; (iii) normalization of liver function and prevention of hepatic complications of HBV; and (iv) permanent eradication of HBV. Because of the involvement of immune complexes in the disease, immunosuppressive therapy—similar to that used in the idiopathic form of the disease—was once fashionable. Corticosteroids were reported to provide symptomatic relief in isolated cases. The contemporary view, however, is that steroid and cytotoxic agents can cause deleterious hepatic flares or even fatal decompensation by enhancing viral replication when the drugs are withdrawn.31
Another approach is treatment with an antiviral agent. Interferon-α is a naturally occurring cytokine produced by B lymphocytes, null lymphocytes, and macrophages that exerts antiviral, antiproliferative and immunomodulatory effects. While reportedly useful in children,32 interferon-α has produced mixed results in adults with HBV-associated membranous nephropathy.21,26
Introduction of the nucleoside analog lamivudine has revolutionized the treatment of chronic HBV infection.33 Lamivudine is the (–)-enantiomer of 3′-thiacytidine. This analog inhibits DNA synthesis by terminating the nascent proviral DNA chain through interference with the reverse transcriptase activity of HBV. In children and adults with HBV-associated membranous nephropathy, lamivudine has been anecdotally reported to induce remission of nephrotic syndrome and to suppress viral replication.24,34 In a recent analysis comparing 10 adult nephrotic patients with HBV-related membranous nephropathy who received lamivudine with 12 matched historical control subjects who presented in the pre-lamivudine era, lamivudine significantly improved proteinuria, aminotransferase levels, and renal outcome over a 3-year period.25 Randomized studies in a larger cohort of patients are needed to prove this effect.
A potential limitation of prolonged treatment with lamivudine is emergence of drug-resistant virus strains resulting from induction and selection of HBV variants with mutations at the tyrosine-methionine-aspartate-aspartate (YMDD) motif of DNA polymerase. One agent that might be useful in lamivudine-resistant cases is adefovir dipivoxil, an acyclic nucleotide analog that is effective against both lamivudine-resistant HBV mutants and wild-type HBV.35 This agent does have nephrotoxic potential, and there are no clinical data on its efficacy in HBV-related membranous nephropathy that does not respond to lamivudine treatment. Data do indicate, however, that the recommended dose of 10 mg adefovir dipivoxil is associated with a relatively low risk of nephrotoxicity.36
While awaiting an ideal agent for treatment of HBV-associated glomerulopathy, active immunization remains the most effective means of immunoprophylaxis.37 In Taiwan, active immunization of all newborns since 1984 has led to a dramatic (10-fold) decline in the incidence of neonatal HBV infection and its sequelae.38 In the US, universal vaccination of infants began in 1991, and a 67% reduction in HBV infection was recorded 10 years later. In 2003, the WHO recommended that all countries establish universal HBV immunization programs for infants and adolescents.39
Glomerulonephritis associated with hepatitis C virus
HCV is a small RNA virus in the Flaviviridae family. Evolution of HCV has been characterized by the emergence of six major genotypes (based on sequence homology) and more than 50 subtypes. To date, around 170–200 million individuals worldwide are estimated by the WHO to be chronically infected with HCV.
Although viral replication is primarily confined to the liver, a variety of extrahepatic disease manifestations are associated with HCV infection. The principal renal manifestation of HCV infection is MCGN type I, usually in the context of type II (mixed) cryoglobulinemia.40 The prevalence of MCGN in HCV type II cryoglobulinemia is approximately 30%. MCGN is occasionally observed in patients with hepatitis C in the absence of cryoglobulinemia.40 Type II MCGN (e.g. dense deposit disease) has not been described in association with HCV infection.
Two immunologic features of HCV might underlie predisposition to extrahepatic disease manifestations. First, HCV is known to evade immune elimination, leading to chronic infection and accumulation of circulating immune complexes. MCGN associated with HCV infection might result from this phenomenon. Second, HCV stimulates production of monoclonal rheumatoid factors. This feature causes type II cryoglobulinemia, which accounts for most symptomatic cryoglobulinemic vasculitis. Although this manifestation occurs relatively infrequently, as do all the extrahepatic disease manifestations, it accounts for much of the increased morbidity and mortality that accompanies the disease.
Between 35% and 90% of HCV-infected patients have been reported to have mixed cryoglobulinemia.41,42 Prevalence increases with duration of hepatitis. It should be noted, however, that the prevalence of mixed cryoglobulinemia has not been determined in populations of unselected HCV-infected patients. Frank symptomatic cryoglobulinemia affects 1% or less of patients and is usually associated with high levels of rheumatoid factor and cryoglobulins. Testing of unselected patients with cryoglobulinemia has shown that up to 90% have anti-HCV antibody. Type I MCGN has long been regarded as idiopathic, but a considerable proportion of patients has concomitant chronic HCV infection. The exact proportion of patients with type I MCGN who are anti-HCV-antibody-positive is unknown.
The true prevalence of MCGN without detectable cryoglobulinemia is difficult to assess. Such cases might represent a subclinical form of cryoglobulinemia in which circulating cryoglobulins have not been detected by standard laboratory techniques. Further, production of IgM antibodies with anti-IgG activity might induce formation of immune complexes that lack cryoprecipitable properties.43 Finally, these patients might develop detectable circulating cryoglobulinemia only later in the course of the disease.44
Diagnosis
Laboratory testing coupled with renal biopsy establishes the diagnosis of HCV-related MCGN. Most patients will have anti-HCV antibody, as well as HCV RNA, in serum. Serum transaminase levels are elevated in 70% of patients. Cryoglobulins are detected in 50–70% of patients. Serum electrophoresis and immunofixation detects type II (mixed) cryoglobulins. Monoclonal rheumatoid factor, almost invariably an IgMκ, is a distinguishing feature of cryoglobulinemic glomerulonephritis. The amount of cryoglobulins, usually measured as a cryocrit, varies between patients and with time in a given patient (range 2–70%). κ Light chains are also commonly present in the urine. The serum complement pattern, which does not change greatly with clinical disease activity, is also discriminative. Characteristically, early complement components (C4 and C1q) and CH50 are present at very low or undetectable levels in these patients, whereas the C3 level tends to remain normal or is only slightly depressed.
Clinical characteristics
Renal disease associated with HCV is rare in children. The typical age of disease onset is the fifth or sixth decade of life after longstanding infection, often in association with mild subclinical liver disease. Patients might have other symptoms of cryoglobulinemia, such as palpable purpura and arthralgias. Renal manifestations include nephrotic (20%) or non-nephrotic proteinuria and microscopic hematuria.45 Acute nephritic syndrome is the presenting feature in about a quarter of cases. Renal insufficiency, frequently of mild severity, occurs in about half of patients. Over 80% of patients have refractory hypertension at presentation, which might be responsible for a considerable number of cardiovascular deaths.
The clinical course of HCV-associated renal disease can vary dramatically. This disease does not frequently progress to uremia, despite the persistence of urinary abnormalities in the majority of patients. When such progression does occur, it tends to be in males and those of older age. According to an Italian series, around 15% of patients eventually require dialysis.46 Other forms of glomerular injury, including membranous nephropathy, FSGS, mesangial proliferative glomerulonephritis, and crescentic glomerulonephritis, have been reported in HCV carriers as individual case reports and small series. Notably, membranous nephropathy in HCV carriers is characterized by the absence of cryoglobulin and male predominance.47
Treatment
In general, therapy can be directed at two levels: removal of cryoglobulins by plasmapheresis; and inhibition of cryoglobulin synthesis by attenuating immune responses (using steroids or cytotoxic agents) or suppressing viral replication (using interferon and ribavirin). Before the association between HCV and cryoglobulinemic MCGN was established, steroids and cyclophosphamide were the mainstays of treatment. Our awareness of this link has facilitated a more rational approach to management of this condition. Controlled trials have shown that antiviral therapy with interferon-α is associated with improvements in systemic symptoms of immune complex disease. Unfortunately, post-therapy relapse occurs in a large proportion of patients, particularly when interferon monotherapy is administered in short courses. Introduction of combination therapy with interferon-α2b plus ribavirin was an important milestone in the treatment of chronic hepatitis C.48 This cocktail has also produced favorable results in mixed cryoglobulinemia, although non-responses and relapses after initial improvements still occur.49
The introduction of pegylated forms of interferon (peginterferon) in 2000 was another breakthrough in treatment of chronic hepatitis C. Recent data on peginterferon and ribavirin combination therapy are encouraging.50 An increased rate of treatment failure in carriers of HCV genotype 1 has been recognized.51 Observational studies support the effectiveness of peginterferon and ribavirin combination therapy in HCV-associated cryoglobulinemic MCGN. One therapeutic drawback is the hemolytic effect that complicates ribavirin therapy, particularly in patients with functional renal impairment. This difficulty has been overcome by adjusting the dose according to glomerular filtration rate instead of body weight alone, and utilizing recombinant erythropoietin to combat anemia. Post-treatment renal biopsy showed histological improvement in two of three patients who received combination therapy for 12 months.52
HIV-related glomerular diseases
The wide spectrum of glomerulopathies occurring in the course of HIV infection can be classified into four groups. The first group is the classical HIV-associated nephropathy (HIVAN), a distinct entity with histological features of FSGS with tuft collapse or, more rarely, mesangial hyperplasia. HIVAN seems to be related to a direct effect of HIV or viral proteins on renal epithelium.53 The second group is a diffuse proliferative-mesangiocapillary or lupus-like glomerulonephritis, with predominantly mesangial immune deposits, also known as HIV immune-complex-mediated disease.54 This group also includes other immune-complex-mediated glomerulonephritides with more-heterogeneous histological features. The third group (which includes immunotactoid glomerulonephritis) is heterogeneous with regard to glomerular lesions and pathogenic mechanisms, some of which are still undefined. The true role of HIV infection in glomerulopathies of this type is also uncertain. The final group includes HIV-associated thrombotic microangiopathy/hemolytic uremic syndrome, in which HIV is the main, but not sole, etiological factor. Ethnic/geographic background is an important determinant of the type of glomerulopathy associated with HIV; for example, collapsing FSGS is prevalent in patients of African descent.
The FSGS variant of HIVAN is the most commonly reported chronic renal disease associated with HIV infection. HIVAN affects up to 10% of HIV-infected patients of African descent—mainly males at risk of drug abuse, and often African Americans. Glomerular changes associated with this variant are capillary wall collapse of varying severity, with widening of Bowman's space. Visceral epithelial cells undergo hyperplasia and hypertrophy, and develop protein inclusions in their swollen cytoplasm surrounding the collapsed lobules. Sclerosis affects segments of capillary tuft or the whole glomerular surface. Tubular cells might undergo degenerative changes, necrosis or flattening. Large dense casts can develop in dilated tubules.
Detection of HIV RNA in renal tissue from patients with HIVAN, and of HIV DNA in patients with and without nephropathy,55 raises the question of whether renal cells can be infected in vivo (tubular epithelial cells can be infected in vitro).4 Renal uptake of viral gene products might induce transactivation of host genes. In renal cells, HIV proteins can cause apoptosis,4 phenotypic modifications,56 and subsequent tubulointerstitial fibrosis.
Clinical characteristics
Clinical manifestations of HIVAN with FSGS are nephrotic-range proteinuria and renal insufficiency. Hypertension and edema are uncommon. In overt cases, ultrasonography typically reveals enlarged, highly echogenic kidneys, which probably develop in response to microcystic tubular dilatation. Before effective antiretroviral treatment was available, clinical progression was rapid. Intensive antiretroviral treatment delays progression.57
Pathology of the HIV-associated disease mediated by immune complexes resembles lupus nephropathy. The clinical presentation is nephrotic syndrome with microscopic hematuria. Progression to renal failure occurs, but more slowly than in HIVAN. Patients often have HCV coinfection, but HIV seems to have the prevailing role. Viral antigen has been detected in glomeruli, and antibodies eluted from the kidney react with HIV antigens in circulating immune complexes (IgA-p24 antigen, IgG-p24 and IgG-gp120). In cases of HIV-related IgAN, circulating immune complexes containing IgA idiotype antibodies have been detected.58
Most patients with HIV-associated thrombotic microangiopathy/hemolytic uremic syndrome present with acute renal failure, microscopic hematuria, and non-nephrotic proteinuria. Multiorgan involvement is frequent and prognosis is poor, with a high rate of mortality. Multifactorial etiologies encompass drugs, neoplasia, lymphoma and infection.
Parvovirus B19
PVB19 has been associated with acute glomerulonephritis. Typical life stage of onset is the second or third decade. Patients present with mild proteinuria and microhematuria, with low levels of serum complement 3. The pathology of this disease is characterized by endocapillary glomerulonephritis, MCGN, or both, with subendothelial deposits. PVB19 capsid protein is found in the glomeruli.10 Spontaneous recovery is the norm.
PVB19 is also associated with collapsing glomerulopathy. Prevalence of PVB19 DNA in renal biopsies (78%) and peripheral blood (87%) is significantly higher in patients with collapsing glomerulopathy than in those with other nephropathies.10 Glomerular and tubular infection with PVB19 might trigger collapsing glomerulopathy, but only in patients with immune defects and a racial predisposition (African descent).8 Most intriguingly, Tanawattanacharoen and coworkers9 detected PVB19 DNA in 80% of patients with 'idiopathic' FSGS, and frequently also in controls. These results possibly reflect the presence of latent DNA from past infection. Failure to localize PVB19 nucleic acid within kidney is evidence against ongoing, high-level viral replication.
Hantavirus
Hantaviruses are responsible for 'hemorrhagic fever with renal syndrome', an acute interstitial nephritis resulting from direct vascular injury of renal tissue.59 The severe form leads to acute renal failure in 50% of cases. Less severe forms occur in nonendemic areas, and present primarily as fever, hepatitis, and mild renal impairment. In most cases, glomeruli are not affected and the pathology is tubulointerstitial. Isolated cases with immune complex glomerular disease have been described, in association with diffuse proliferative glomerulonephritis and complete recovery after remission of the systemic clinical syndrome.60
Severe acute respiratory syndrome coronavirus
Six percent of patients suffering from severe acute respiratory distress syndrome had acute renal impairment.61 Despite detection of viral DNA in the urine, there was no evidence of viral tropism of the kidney. The pathology is exclusively tubulointerstitial nephritis. The mechanism of disease is probably related to multiorgan failure, rhabdomyolysis, and hemodynamic disturbance.
Other viruses
Hepatitis A virus infection can present as acute post-infectious glomerulopnephritis with pathology resembling that of IgAN.64 More commonly, hepatitis A virus induces acute renal failure secondary to acute fulminant hepatitis.65 Acute renal failure complicating other viral infections, such as Epstein–Barr virus66 and dengue fever,67 is related to multiorgan failure, rhabdomyolysis, and hepatorenal syndrome.
Conclusion
Diverse mechanisms of glomerular and tubulointerstitial injury and heterogeneous clinicopathologic patterns underlie the relationship between viral infection and glomerular disease. The etiological role of some viruses is still undefined. Molecular biology techniques are vital in elucidating the precise location and role of viruses in the pathogenesis of virus-related nephropathy.
Review criteria
We searched PubMed using the term “virus” with the following keywords in different combinations: “glomerulonephritis”, “nephropathy”, “renal failure”, and “kidney”.
References
Glassock RJ (1991) Immune complex-induced glomerular injury in viral diseases: an overview. Kidney Int 40 (Suppl 35): S5–S7
Couser WG (1985) Mechanisms of glomerular injury in immune-complex disease. Kidney Int 28: 569–583
Golbus SM and Wilson C (1979) Experimental glomerulonephritis induced by in-situ formation of immune complexes in glomerular capillary wall. Kidney Int 16: 148–157
Conaldi PG et al. (1998) HIV-1 kills renal tubular epithelial cells in vitro by triggering an apoptotic pathway involving caspase activation and Fas upregulation. J Clin Invest 102: 2041–2049
Segerer S et al. (2000) Chemokines, chemokine receptors, and renal disease: from basic science to pathophysiologic and therapeutic studies. J Am Soc Nephrol 11: 152–176
Glassock RJ et al. (1990) Human immunodeficiency virus infection and the kidney. Ann Intern Med 112: 35–39
D'Amico G (1998) Renal involvement in hepatitis C infection: cryoglobulinemic glomerulonephritis. Kidney Int 54: 650–671
Moudgil A et al. (2001) Association of parvovirus B19 infection with idiopathic collapsing glomerulopathy. Kidney Int 59: 2126–2133
Tanawattanacharoen S et al. (2000) Parvovirus B19 DNA in kidney tissue of patients with focal segmental glomerulosclerosis. Am J Kidney Dis 35: 1166–1174
Nakazawa T et al. (2000) Acute glomerulonephritis after human parvovirus B19 infection. Am J Kidney Dis 35: E31
Monath TP (1987) Yellow fever: a medically neglected disease: report on a seminar. Rev Infect Dis 9: 165–175
Ozkutlu S et al. (1989) Fatal mumps myocarditis. Jpn Heart J 30: 109–114
Lin CY and Hsu HC (1983) Measles and acute glomerulonephritis. Pediatrics 71: 398–401
Vas SI (1991) Primary and secondary role of viruses in chronic renal failure. Kidney Int 40 (Suppl 35): S2–S4
Toth T and Szucs S (1988) Focal-segmental glomerulosclerosis associated with herpes simplex viral encephalitis. Child Nephrol Urol 89: 173–175
Levy M and Chen N (1991) Worldwide perspective of hepatitis B-associated glomerulonephritis in the 80s. Kidney Int 40 (Suppl 35): S24–S33
Lai KN and Lai FM (1991) Clinical features and the natural course of hepatitis B virus-related glomerulopathy in adults. Kidney Int 40 (Suppl 35): S40–S45
Lin CY (1991) Clinical features and natural course of HBV-related glomerulopathy in children. Kidney Int 40 (Suppl 35): S46–S53
Lai KN et al. (1988) Strong association between IgA nephropathy and hepatitis B surface antigenemia in endemic areas. Clin Nephrol 29: 229–234
Peters DN et al. (1992) Immunopathology of glomerulonephritis associated with chronic woodchuck hepatitis virus infection in woodchucks. Am J Pathol 141: 143–152
Lai KN et al. (1991) Membranous nephropathy related to hepatitis B virus in adults. N Engl J Med 324: 1457–1463
Ho SK et al. (1999) Comparison of the second-generation digene hybrid capture assay with the branched-DNA assay for measurement of hepatitis B virus DNA in serum. J Clin Microbiol 37: 2461–2465
Tang S et al. (1999) Early detection of hepatocellular carcinoma in hepatitis-B-positive renal transplant recipients. J Surg Oncol 72: 99–101
Lai KN (2001) Hepatitis B associated renal disease. In: Rheumatology and the Kidney, 377–396 (Eds Adu D et al.) Oxford: Oxford University Press
Tang S et al. (2005) Lamivudine in hepatitis B-associated membranous nephropathy. Kidney Int 68: 1750–1758
Conjeevaram HS et al. (1995) Long-term outcome of hepatitis B virus-related glomerulonephritis after therapy with interferon alfa. Gastroenterology 109: 540–546
Lai FM et al. (1994) Primary glomerulonephritis with detectable glomerular hepatitis B virus antigens. Am J Surg Pathol 18: 175–186
Janssen HL et al. (2004) Polyarteritis nodosa associated with hepatitis B virus infection: the role of antiviral treatment and mutations in the hepatitis B virus genome. Eur J Gastroenterol Hepatol 16: 801–807
Tang S et al. (1999) Skin lesions, hepatitis, and nephropathy in a 30-year-old man. Am J Kidney Dis 34: 380–383
Ozdamar SO et al. (2003) Hepatitis-B virus associated nephropathies: a clinicopathological study in 14 children. Pediatr Nephrol 18: 23–28
Lai KN et al. (1990) The therapeutic dilemma of the usage of corticosteroid in patients with membranous nephropathy and persistent hepatitis B virus surface antigenaemia. Nephron 54: 12–17
Lin CY (1995) Treatment of hepatitis B virus-associated membranous nephropathy with recombinant alpha-interferon. Kidney Int 47: 225–230
Lai CL et al. (1998) A one-year trial of lamivudine for chronic hepatitis B: Asia Hepatitis Lamivudine Study Group. N Engl J Med 339: 61–68
Connor FL et al. (2003) HBV associated nephrotic syndrome: resolution with oral lamivudine. Arch Dis Child 88: 446–449
Perrillo R et al. (2000) Adefovir dipivoxil for the treatment of lamivudine-resistant hepatitis B mutants. Hepatology 32: 129–134
Yuen MF and Lai CL (2004) Adefovir dipivoxil in chronic hepatitis B infection. Expert Opin Pharmacother 5: 2361–2367
Poland GA and Jacobson RM (2004) Clinical practice: prevention of hepatitis B with the hepatitis B vaccine. N Engl J Med 351: 2832–2838
Chang MH et al. (1997) Universal hepatitis B vaccination in Taiwan and the incidence of hepatocellular carcinoma in children: Taiwan Childhood Hepatoma Study Group. N Engl J Med 336: 1855–1859
Duclos P (2003) Safety of immunisation and adverse events following vaccination against hepatitis B. Expert Opin Drug Saf 2: 225–231
Johnson RJ et al. (1993) Membranoproliferative glomerulonephritis associated with hepatitis C virus infection. N Engl J Med 328: 465–470
Pawlotsky JM et al. (1994) Immunological disorders in C virus chronic active hepatitis: a prospective case–control study. Hepatology 19: 841–848
Kayali Z et al. (2002) Hepatitis C, cryoglobulinemia, and cirrhosis: a meta-analysis. Hepatology 36: 978–985
Rennke HG (1995) Secondary membranoproliferative glomerulonephritis. Kidney Int 47: 643–656
Fabrizi F et al. (1998) Hepatitis C virus infection and acute or chronic glomerulonephritis: an epidemiological and clinical appraisal. Nephrol Dial Transplant 13: 1991–1997
D'Amico G (1998) Renal involvement in hepatitis C infection: cryoglobulinemic glomerulonephritis. Kidney Int 54: 650–671
Tarantino A et al. (1995) Long-term predictors of survival in essential mixed cryoglobulinemic glomerulonephritis. Kidney Int 47: 618–623
Uchiyama-Tanaka Y et al. (2004) Membranous glomerulonephritis associated with hepatitis C virus infection: case report and literature review. Clin Nephrol 61: 144–150
Poynard T et al. (1998) Randomised trial of interferon α2b plus ribavirin for 48 weeks or for 24 weeks versus interferon α2b plus placebo for 48 weeks for treatment of chronic infection with hepatitis C virus: International Hepatitis Interventional Therapy Group (IHIT). Lancet 352: 1426–1432
Zuckerman E et al. (2000) Treatment of refractory, symptomatic, hepatitis C virus related mixed cryoglobulinemia with ribavirin and interferon-alpha. J Rheumatol 27: 2172–2178
Fried MW et al. (2002) Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med 347: 975–982
Hadziyannis SJ et al. PEGASYS International Study Group (2004) Peginterferon-α2a and ribavirin combination therapy in chronic hepatitis C: a randomized study of treatment duration and ribavirin dose. Ann Intern Med 140: 346–355
Rossi P et al. (2003) Hepatitis C virus-related cryoglobulinemic glomerulonephritis: long-term remission after antiviral therapy. Kidney Int 63: 2236–2241
Bourgoignie JJ (1990) Renal complications of human immunodeficiency virus type 1. Kidney Int 37: 1571–1584
Kimmel PL et al. (1993) HIV-associated immune-mediated renal disease. Kidney Int 44: 1327–1340
Bruggeman LA et al. (2000) Renal epithelium is a previously unrecognized site of HIV-1 infection. J Am Soc Nephrol 11: 2079–2087
Barisoni L et al. (1999) The dysregulated podocyte phenotype: a novel concept in the pathogenesis of collapsing idiopathic focal segmental glomerulosclerosis and HIV-associated nephropathy. J Am Soc Nephrol 10: 51–61
Eustace JA et al. (2000) Cohort study of the treatment of severe HIV-associated nephropathy with corticosteroids. Kidney Int 58: 1253–1260
Kimmel PL et al. (1992) Brief report: idiotypic IgA nephropathy in patients with human immunodeficiency virus infection. N Engl J Med 327: 702–706
Cosgriff TM and Lewis RM (1991) Mechanism of disease in hemorragic fever with renal syndrome. Kidney Int 40 (Suppl 35): S72–S79
Grcevska L et al. (1990) Different pathohistological presentations of acute renal involvement in Hantan virus infection: report of two cases. Clin Nephrol 34: 197–201
Chu KH et al. (2005) Acute renal impairment in coronavirus-associated severe acute respiratory syndrome. Kidney Int 67: 698–705
Nickeleit V et al. (2000) BK-virus nephropathy in renal transplants—tubular necrosis, MHC-class II expression and rejection in a puzzling game. Nephrol Dial Transplant 15: 324–332
Nebuloni M et al. (1999) BK virus renal infection in a patient with the acquired immunodeficiency syndrome. Arch Pathol Lab Med 123: 807–811
Cheema SR et al. (2004) IgA-dominant glomerulonephritis associated with hepatitis A. Clin Nephrol 62: 138–143
Wilkinson SP et al. (1978) Renal failure in otherwise uncomplicated acute viral hepatitis. Br Med J 2: 338–341
Tsai JD et al. (2003) Epstein–Barr virus-associated acute renal failure: diagnosis, treatment, and follow-up. Pediatr Nephrol 18: 667–674
Lee IK et al. (2005) Clinical characteristics and risk factors for concurrent bacteremia in adults with dengue hemorrhagic fever. Am J Trop Med Hyg 72: 221–226
Acknowledgements
Some of the authors' work cited in this review was supported by the L & T Charitable Fund and INDOCAFE.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Lai, A., Lai, K. Viral nephropathy. Nat Rev Nephrol 2, 254–262 (2006). https://doi.org/10.1038/ncpneph0166
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ncpneph0166
This article is cited by
-
Tumor necrosis factor-α synergistically enhances polyinosinic-polycytidylic acid-induced toll-like receptor 3 signaling in cultured normal human mesangial cells: possible involvement in the pathogenesis of lupus nephritis
Clinical and Experimental Nephrology (2015)
-
Glomerular expression of fractalkine is induced by polyinosinic-polycytidylic acid in human mesangial cells: possible involvement of fractalkine after viral infection
Pediatric Research (2013)
-
Nephrotic syndrome in Kawasaki disease: a report of three cases
Pediatric Nephrology (2012)
-
Hepatitis B virus associated focal and segmental glomerular sclerosis: report of two cases and review of literature
Clinical and Experimental Nephrology (2009)
-
Current status and issues of C1q nephropathy
Clinical and Experimental Nephrology (2009)