Abstract
The aging myopathy manifests itself with diastolic dysfunction and preserved ejection fraction. We raised the possibility that, in a mouse model of physiological aging, defects in electromechanical properties of cardiomyocytes are important determinants of the diastolic characteristics of the myocardium, independently from changes in structural composition of the muscle and collagen framework. Here we show that an increase in the late Na+ current (INaL) in aging cardiomyocytes prolongs the action potential (AP) and influences temporal kinetics of Ca2+ cycling and contractility. These alterations increase force development and passive tension. Inhibition of INaL shortens the AP and corrects dynamics of Ca2+ transient, cell contraction and relaxation. Similarly, repolarization and diastolic tension of the senescent myocardium are partly restored. Thus, INaL offers inotropic support, but negatively interferes with cellular and ventricular compliance, providing a new perspective of the biology of myocardial aging and the aetiology of the defective cardiac performance in the elderly.
Similar content being viewed by others
Introduction
The aging myopathy typically manifests itself with diastolic dysfunction and preserved ejection fraction (EF)1. More than 50% of patients with heart failure have normal or near normal EF and the incidence and prevalence of this condition increases with age. Although the claim is commonly made that age-associated physiological changes predispose older adults to develop heart failure with normal EF, the aetiology of diastolic heart failure is unknown. The difficulty in defining myocardial aging and the mechanisms involved further complicates the recognition of the cellular processes underlying impaired diastolic relaxation. In the current study, we raised the possibility that, in a mouse model of physiological aging, defects in the electromechanical properties of cardiomyocytes are critical determinants of the altered cardiac performance, independently from changes in the structural composition of the myocardium and collagen framework. Cardiomyocytes constitute nearly 90% of the myocardium and alterations in the mechanisms of cell excitation, force generation and kinetics of contraction may be critical in conditioning the abnormalities of the old heart. Aging alters the pattern of electrical activation, resulting in prolonged myocardial repolarization1,2, which enhances the risk of ventricular arrhythmias and sudden death3. Moreover, prolongation of the time of repolarization and perturbations of intracellular ionic balance may influence the contractile and relaxation dynamics of the aging heart, impacting on diastolic and systolic function4.
Impaired diastolic function includes abnormalities in muscle relaxation, distensibility and ventricular filling pattern5. The increase in myocardial stiffness has been ascribed to changes in the composition of the extracellular matrix and/or to intrinsic characteristics of cardiomyocytes6. Switches in titin-isoforms and post-translational modifications of this cytoskeletal protein may affect the passive mechanical properties of myocytes, but whether defective electrical activation contributes to the process remains to be defined.
The mechanical activity of the myocardium is initiated by the electrical excitation of the tissue, which acts as a functional syncytium in propagating membrane depolarization in cardiomyocytes. The upstroke of the action potential (AP) triggers Ca2+ entry via L-type channels, which results in cytosolic Ca2+ rise by the translocation of Ca2+ from the sarcoplasmic reticulum (SR) to the cytoplasm, initiating cell contraction7. The repolarization phase of the AP is a relevant variable of the kinetics of Ca2+ entry; it controls the pattern of Ca2+ influx via L-type channels and Na+/Ca2+ exchanger (NCX) in a time- and voltage-dependent manner7,8,9,10,11,12. Thus, transmembrane Ca2+ fluxes and Ca2+-induced Ca2+ release modulate the temporal dynamics of [Ca2+]i (refs 8, 9, 10, 11, 12), providing an important link between electrical activity, on the one hand, and myocyte and myocardial contractility and relaxation, on the other hand. This possibility has been tested here to document whether defects in this tightly controlled system become apparent in the senescent heart contributing to the manifestations of the aging myopathy and impaired diastolic function.
Here we show that aging leads to an increase in the late Na+ current (INaL), which contributes to the prolongation of the AP duration (APD) and protracted electrical recovery of the aged myocardium. The remodelled electrical properties of cardiomyocytes provide inotropic support to the senescent muscle, but negatively interfere with cellular and myocardial relaxation. Inhibition of INaL partly corrects the defective electrical and diastolic properties of the aged heart. These findings offer a new perspective of the cellular and molecular mechanisms that define the aetiology of the aging myopathy and may have important biological and clinical implications.
Results
Aging impairs ventricular performance
To determine the effects of aging on left ventricular (LV) function, echocardiographic measurements, including speckle-tracking and Doppler protocols, were combined with magnetic resonance imaging (MRI) and invasive haemodynamics. Mice from 3 to 35 months of age were studied. Systolic blood pressure, evaluated by the tail-cuff plethysmography, was comparable in unanaesthetized mice at 3, 25 and 30 months of age, indicating that systemic blood pressure did not increase with aging in this model (Supplementary Fig. 1). EF was preserved at 24 months, when an increase in LV mass was observed. EF slightly decreased at 30 months together with an increase in cavitary volume, whereas stroke volume and cardiac output were maintained (Supplementary Fig. 2). These properties were confirmed by cardiac MRI13 (Fig. 1a,b). In addition, speckle-tracking strain echocardiographic imaging demonstrated that radial and longitudinal strain and strain rates were altered in old mice (Supplementary Fig. 3). Importantly, following blockade of the autonomous nervous system14, differences in EF observed between mice at 3–4 and 30–31 months of age were attenuated (Supplementary Fig. 4), indicating that alterations in cardiac performance with age are partly dictated by an imbalance in autonomic nerve activity, as reported previously15,16.
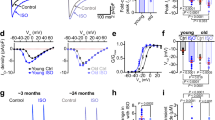
(a) Short axis views by MRI of young (3 months, 3 m) and old (30 months, 30 m) male mouse hearts. (b) Quantitative data obtained by MRI imaging in 3 months (3 m, n=6) and 30 months (30 m, n=5) male mice are shown as mean±s.e.m. and scatter plots. *P<0.05 versus 3 m (Student’s t-test and Mann–Whitney rank sum test); NP, non-parametric analysis. (c) Transmitral flow Doppler echocardiograms obtained in young (3 months, 3 m) and old (30 months, 30 m) male mice show early passive filling (E) and active filling (A) waves. (d) Quantitative data in male mice at 3 months (3 m, n=33), 25 months (25 m, n=19) and 30 months (30 m, n=38) are shown as mean±s.e.m. and scatter plots. *P<0.05 versus 3 m, **P<0.05 versus 25 m (one-way ANOVA with Bonferroni’s post hoc test and Kruskal–Wallis one-way ANOVA on ranks with Dunn’s post hoc test); NP, non-parametric analysis. (e) Haemodynamic and volumetric parameters obtained by pressure–volume (PV) catheterization in male mice. Data in animals at 3–4 months (3–4 m, n=12), 11–12 months (11–12 m, n=14), 24–25 months (24–25 m, n=15) and 30–35 months (30–35 m, n=19) are shown as mean±s.e.m. and scatter plots. LVEDPV, LV end-diastolic PV relation; P, pressure. *P<0.05 versus 3–4 m, **P<0.05 versus 11–12 m, ***P<0.05 versus 24–25 m (one-way ANOVA with Bonferroni’s post hoc test and Kruskal–Wallis one-way ANOVA on ranks with Dunn’s post hoc test); m, months; NP, non-parametric analysis.
To define the diastolic properties of the LV, transmitral flow Doppler echocardiography was employed17. Mice at 25 months of age and older presented reduced passive filling velocity (E-wave), increased active filling (A-wave), lower E/A ratio and prolonged timing parameters (Fig. 1c,d). Haemodynamically, an increase in end-diastolic pressure occurred at 24–25 months and preceded the deterioration in systolic performance; in fact, LV systolic pressure, developed pressure and +dP/dt decreased significantly in mice at 30–35 months of age, although stroke volume and cardiac output were preserved (Fig. 1e). In contrast, reduced −dP/dt, prolonged time constant of pressure decay (τ), and increased end-diastolic pressure were present at 24–25 months, documenting a decline in diastolic function in the absence of systolic defects. In addition, occlusion of the inferior vena cava or aortic arch, which alters LV loading, showed that the slope of the LV end-diastolic pressure–volume (PV) relation was 2.5-fold steeper in old than in young animals, pointing to alterations in ventricular compliance with age (Fig. 1e). The deterioration of diastolic and systolic haemodynamic indices in mice ≥30 months was also observed following complete autonomic blockade (Supplementary Fig. 5). Thus, these in vivo data emphasize the defective kinetics of contraction and relaxation, and the impaired diastolic filling of the old heart, which precede, chronologically, the compromise of systolic function.
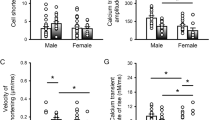
LV weight was similar in mice at 3 and 11 months of age, and increased modestly at 23–25 months. Myocardial hypertrophy became apparent in the senescent heart at 28–34 months (Supplementary Fig. 6a), when cardiac performance was depressed. Structurally, cardiomyocytes were 34% larger in senescent mice; cellular hypertrophy was associated with changes in the expression of fibrotic markers18 and a relatively modest increase in myocardial interstitial fibrosis, from 0.2% at 2–3 months of age to 0.3% at 29–30 months (Supplementary Fig. 6b–e). Collectively, these results support the notion that the aging myopathy is characterized by an initial decline of diastolic function, dictated by protracted kinetics of contraction and relaxation. These alterations are later accompanied by intervening defects in systolic performance, and myocyte and myocardial hypertrophy.
Aging delays myocardial and myocyte electrical recovery
Surface electrocardiogram (ECG) was employed to assess the electrical properties of the heart in mice at 3, 11–13, 25 and 30–34 months of age. PR and QT intervals were prolonged in mice ≥25 months (Fig. 2a,b), indicating that delays in atrioventricular conduction and ventricular repolarization occurred with age. These changes were also observed by telemetry in conscious animals and persisted following autonomic blockade (Supplementary Figs 7 and 8). To exclude the confounding effects of circulating neurohumoral factors, an isolated organ preparation was employed12. Again, old hearts showed delayed PR and QT intervals (Fig. 2c). In addition, epicardial monophasic APs (MAPs), which reflect local cellular depolarization and repolarization, were recorded on the LV free wall12. APD was prolonged in old hearts (Fig. 2d,e), suggesting that defects in the electrical properties of the aging myocardium were mediated by alterations at the cellular level. Since repolarization delays are associated with electrical disturbances3,12, the propensity of the aging heart to develop arrhythmias was assessed by a protocol of programmed electrical stimulation (PES)12. Arrhythmic events were 2.6-fold more frequent in hearts ≥24 months of age (Supplementary Fig. 9).
(a) ECGs from a young (3 months, 3 m; black traces) and old (25 months, 25 m; blue traces) mouse. Scale bars, 200 ms, 0.2 mV. Superimposed traces are shown in the inset. Scale bars, 50 ms, 0.2 mV (b,c) Electrocardiographic parameters in anaesthetized male mice (b) and explanted hearts (c) at 3–4 (∼3 m, n=28,13), 11–13 (∼12 m, n=13,16), 24–27 (∼25 m, n=12,15) and 30–34 months (∼30 m, n=20,12) are mean±s.e.m. and scatter plots. *P<0.05 versus ∼3 m, **P<0.05 versus ∼12 m (one-way ANOVA with Bonferroni’s post hoc test and Kruskal–Wallis one-way ANOVA on ranks with Dunn’s post hoc test); NP, non-parametric analysis. (d) Epicardial monophasic action potentials (MAPs) in perfused hearts from male mice at 3 (3 m; black traces) and 30 months (30 m; blue traces). Scale bars, 200 ms, 4 mV. Superimposed traces are shown in the inset. Scale bars, 100 ms, 2 mV (e) Data at sinus rhythm (Sinus) or 8 Hz stimulation (Paced) in hearts from male mice at 3–4 (∼3 m, n=11), 11–13 (∼12 m, n=16), 24–27 (∼25 m, n=15) and 30 months (30 m, n=11) are mean±s.e.m. and scatter plots. APD50, APD90: MAP duration at 50 and 90% repolarization, respectively. *P<0.05 versus ∼3 m, **P<0.05 versus ∼12 m (one-way ANOVA with Bonferroni’s post hoc test and Kruskal–Wallis one-way ANOVA on ranks with Dunn’s post hoc test); NP, non-parametric analysis. (f) APs recorded in LV myocytes from young (black traces) and old (blue traces) mice. Scale bars, 300 ms, 20 mV. Superimposed traces are shown in the inset. Scale bars, 50 ms, 20 mV. (g) AP parameters of myocytes from male mice at 3 (Young, n=50 cells from 18 hearts) and 26–30 months (Old, n=42 cells from 23 hearts) are mean±s.e.m. RMP, resting membrane potential. *P<0.05 versus Young (Mann–Whitney rank sum test). Scattered plots are reported in Supplementary Fig. 10. m, months.
The electrical properties of isolated LV myocytes were established by the patch-clamp technique11,12. The duration of the AP, measured at 90% (APD90) of repolarization, was 1.8-fold longer in 26–30-month-old myocytes (Fig. 2f,g and Supplementary Fig. 10). The changes in APD detected in old myocytes were consistent with the 24% reduction in the maximal negative slope during repolarization (dV/dtmin). However, resting membrane potential, AP amplitude and dV/dtmax were comparable in myocytes at all ages. These electrical parameters, obtained at 1 Hz pacing rate, were maintained at higher stimulation frequency (Supplementary Fig. 11). Collectively, myocardial aging is characterized by electrical disturbances and the prolongation of the AP in myocytes contributes to the delayed electrical recovery of the old heart.
Aging alters transmembrane ionic currents
Voltage-gated outward K+ Kv currents are primary determinants of the AP repolarization and major factors in the electrophysiological remodelling occurring in myocytes during maturation, cardiac pathology and aging8,19,20,21,22,23. Kv currents comprise components with rapid activation and fast (Ito), intermediate (IK,slow1) and slow (IK,slow2) kinetics of inactivation, together with a sustained (non-inactivating) component (Iss)21,24. By voltage-clamp protocols24, Kv currents were reduced in LV myocytes from old mice, at 31–32 months of age (Fig. 3a,b and Supplementary Fig. 12). Thus, reduction of Kv currents in old myocytes contributes partly to the prolonged repolarization of the AP.
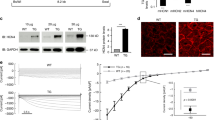
(a) Whole-cell voltage-gated K+ currents recorded in voltage-clamp in a young (black traces) and an old (blue traces) LV myocyte. Scale bars, 3 s, 10 pA pF−1. Voltage-clamp protocol is reported in the lower traces. (b) Quantitative data for the amplitude of Kv current components in myocytes from mice at 3–4 months (Young, n=14 cells from 5 hearts) and 31–32 months (Old, n=19 cells from 4 hearts) are shown as mean±s.e.m. *P<0.05 versus Young (Student’s t-test and Mann–Whitney rank sum test); NP, non-parametric analysis. (c) Whole-cell voltage-gated Na+ currents recorded in voltage-clamp in a young (black traces) and an old (blue traces) LV myocyte. Scale bars, 100 ms, 0.6 pA pF−1. The voltage-command protocol, which is shown in the lower traces, comprised a preconditioning pulse to +50 mV to prevent Na+ influx and minimize loss of voltage control. Alternative voltage-clamp protocols were also employed (see Supplementary Figs 13 and 14). (d) Quantitative data in myocytes from male mice at 3 months (Young, n=31 cells from 4 hearts) and 30 months (Old, n=30 cells from 4 hearts) are shown as mean±s.e.m. and scatter plots. *P<0.001 versus Young (Mann–Whitney rank sum test).
Next, we tested whether changes in the slowly inactivating late Na+ (INaL) and L-type Ca2+ (ICaL) inward currents were implicated in the protracted AP. INaL current density was 1.6-fold higher in 30-month-old cardiomyocytes than in 3-month-old cells (Fig. 3c,d). In addition, INaL maximal conductance was increased, whereas voltage activation and steady-state inactivation were comparable in young and old cells (Supplementary Fig. 13). Moreover, we dissected pharmacologically INaL in young and old myocytes using tetrodotoxin (TTX) and found that the TTX-sensitive slowly inactivating current was increased in old cardiomyocytes (Supplementary Fig. 14). The fast inactivating Na+ current (INa) that controls the upstroke of the AP was not altered with aging (Supplementary Fig. 15), a result which is consistent with the preservation of the amplitude and upstroke velocity of the AP. Similarly, the density and properties of ICaL were comparable in young and old cells (Supplementary Fig. 16).
The role of INaL in myocyte repolarization was determined by measuring the AP before and after inhibition of this current. We employed ranolazine, a selective inhibitor of INaL (refs 25, 26, 27), and mexiletine, a blocker of the late Na+ current that, as previously reported28,29, minimally affects peak INa (Supplementary Fig. 17). Reduction of INaL shortened the intermediate and late repolarization phases of the AP in old myocytes, but had an attenuated impact on young cells (Fig. 4a–d and Supplementary Fig. 18). Moreover, the duration of LV MAP measured at 90% repolarization was reduced following mexiletine perfusion by 14 and 22% in 5- and 24–30-month-old hearts, respectively (Supplementary Fig. 19), indicating that inhibition of INaL abbreviates the repolarization phase of the AP.
(a) APs recorded in an old myocyte before (Tyrode, black traces) and after exposure to the Na+ channel inhibitor mexiletine (Mex, red traces). Scale bars, 200 ms, 20 mV. Superimposed traces are reported in the inset. Scale bars, 500 ms, 20 mV. (b) Quantitative data in myocytes from male mice at 3 months (Young, n=10 cells from 5 hearts) and 27–30 months (Old, n=8 cells from 5 hearts) before (Tyr) and after exposure to 10 μM mexiletine (Mex) are shown as mean±s.e.m. and scatter plots. Tyr, Tyrode solution. *P<0.05 versus Tyr (paired t-test and Wilcoxon signed rank test); NP, non-parametric analysis. Additional parameters are reported in Supplementary Fig. 18. (c) APs recorded in an old myocyte before (Tyrode, black traces) and after exposure to INaL inhibitor ranolazine (Ran, red traces). Scale bars, 300 ms, 20 mV. Superimposed traces are reported in the inset. Scale bars, 50 ms, 20 mV. (d) Quantitative data in myocytes from mice at 3–6 months (Young, n=14 cells from 4 hearts) and 27–33 months (Old, n=8 cells from 5 hearts) before (Tyr) and after exposure to 10 μM ranolazine (Ran) are shown as mean±s.e.m. and scatter plots. *P<0.05 versus Tyr (paired t-test). Additional parameters are reported in Supplementary Fig. 18. (e) Quantitative data for electrocardiographic parameters obtained in male mice at 3–4 months (Young) and at 30–31 months (Old) at baseline (Base) and 1 h after treatment with saline (Young, n=9; Old n=8) or ranolazine (30 mg per kg body weight, i.p.) (Young, n=8; Old n=8). Data are shown as mean±s.e.m. and scatter plots. *P<0.05 versus Base (paired t-test and Wilcoxon signed rank test); NP, non-parametric analysis.
To establish whether INaL was implicated in the prolongation of the QT interval in the senescent heart (see Fig. 2b), mice were treated systemically with an inhibitor of INaL, and ECGs were recorded at baseline and 1 h after administration of the inocula. In young animals, inhibition of INaL had no major consequences on the electrical recovery of the myocardium. Conversely, blockade of INaL in old mice with ranolazine or mexiletine shortened the QT interval by 7% and 8%, respectively (Fig. 4e and Supplementary Fig. 20a). These results were confirmed in conscious mice by telemetry (Supplementary Fig. 20b). Thus, alterations in INaL prolong myocyte AP and electrical recovery of the senescent myocardium, and inhibition of INaL partially corrects this defect, restoring in vivo a younger cardiac phenotype.
Electrical activity modulates Ca2+ transients in myocytes
The repolarization of the AP controls the transmembrane Ca2+ influx via L-type channels and the NCX, which, in turn, activates the release of Ca2+ from the SR7,8,9,10,11,12. Thus, we tested whether the protracted electrical repolarization of the AP in senescent myocytes was associated with alterations in Ca2+ cycling and cell contractility. Ca2+ transients and unloaded cell shortening were evaluated in young, 3 months, and old, 29–30 months, myocytes. At 1 Hz, Ca2+ transient amplitude and cell shortening were similar in both groups of myocytes, but time to peak Ca2+ transient was delayed by 26%, and time to peak shortening was prolonged by 21% in old cells. Moreover, Ca2+ decay and myocyte relengthening at 90% were, respectively, 54% and 32% longer in old myocytes. The delay in the timing parameters was maintained at higher stimulation frequencies, although a reduction in developed Ca2+ was observed (Fig. 5a–d, Supplementary Fig. 21).
(a) Ca2+ transients of young (black traces) and old (blue traces) myocytes. Scale bars, 1 s, 0.25 F/F0. Superimposed traces are reported in the inset. Scale bars, 250 ms, 0.25 F/F0. (b) Quantitative data for Ca2+ transients obtained in myocytes from mice at 3 months (Young) and 29–30 months (Old) (Young, n=195 cells from 16 hearts; Old, n=131 cells from 11 hearts); data are shown as mean±s.e.m. and scatter plots. *P<0.05 versus Young (Mann–Whitney rank sum test). (c) Cell shortening of young (black traces) and old (blue traces) myocytes. Scale bars, 1 s, 1 μm. Superimposed traces are reported in the inset. Scale bars, 250 ms, 1 μm (d) Quantitative data for cell shortening properties obtained in myocytes from mice at 3 months (Young) and 29–30 months (Old) (Young, n=129 cells from 8 hearts; Old, n=117 cells from 7 hearts); data are shown as mean±s.e.m. and scatter plots. *P<0.05 versus Young (Mann–Whitney rank sum test). (e) AP profiles (upper traces) obtained from myocytes at different age (m, months) were applied as voltage-clamp command (AP-clamp) in an old myocyte to elicit Ca2+ transients (lower traces). Scale bars, 300 ms, 1 F/F0, 40 mV. Ionic currents recorded are omitted. (f) Decay of Ca2+ transients elicited by five different AP-clamp protocols obtained in myocytes from male mice at 3–5 months (Young) and 28–33 months (Old) are shown as mean±s.e.m. (Young, n=12 cells from 5 hearts; Old, n=9 cells from 2 hearts). Levels of the Ca2+ fluorescence signal from the peak of the Ca2+ transient to 90% of the decay are reported. Data were fitted with mono-exponential functions (solid lines). Fitting parameters are reported in Supplementary Table 3. (g) Bivariate plot for APD50 of the AP used as Vclamp command and Ca2+ transient properties obtained from young and old myocytes reported in f. Data were fitted with linear regressions (dashed lines). Fitting parameters are reported in Supplementary Table 3.
To establish a causative link between the prolongation of the AP in old cardiomyocytes and the delayed kinetics and preserved amplitude of Ca2+ transients, AP-clamp experiments were performed. Two short APs (APD50: 5 and 7 ms) collected from two young myocytes and three longer APs (APD50: 17, 26 and 80 ms) obtained from three old cells were employed as voltage-clamp command while monitoring intracellular Ca2+ levels. As previously reported11,12,30, longer APD led to a progressive increase in Ca2+ transient amplitude, but the slope of the positive APD-inotropic relationship was attenuated in old myocytes (Fig. 5e–g). In young and old cardiomyocytes, increases in APD resulted in delayed time to peak Ca2+ and Ca2+ decay at 30%; however, the late decay, measured at 90%, negatively correlated with APD. When the same AP shape was imposed to young and old myocytes, the timing parameters of Ca2+ transients were comparable in young and old cells, although Ca2+ transient amplitude tended to be reduced in senescent myocytes (Fig. 5f and Supplementary Fig. 22). These results suggest that (i) prolongation of the AP increases the amplitude of Ca2+ transients in cardiomyocytes, but the efficiency of the process is attenuated in old cells; (ii) longer durations of the AP delay time to peak and early decay of Ca2+ transients; and (iii) experimental conditions, including cell dialysis, abolish the slower Ca2+ transient kinetics observed in old cells with field stimulation.
The lack of potentiated Ca2+ transient amplitude in old myocytes with protracted APD raised the possibility that SR Ca2+ load was affected with age, a factor that may limit Ca2+ release following electrical excitation31. Field-stimulated myocytes were rapidly exposed to caffeine to assess SR Ca2+ content11,12,32. The amplitude of the AP-induced Ca2+ transient was comparable in young and old cells but the caffeine-induced Ca2+ release was reduced in old myocytes (Fig. 6a,b), suggesting a defective SR Ca2+ load. The decay of the caffeine-induced Ca2+ transient that reflects NCX activity33 was faster in old myocytes (Fig. 6c). Thus, a reduced SR Ca2+ load in old myocytes may condition the attenuated amplitude of the Ca2+ transients in spite of a prolongation of the AP.
(a) Line scan image of a young myocyte loaded with the Ca2+ indicator Fluo-4 (green). Ca2+ transients were elicited by electrical pacing or by 20 mM caffeine spritz (arrow). Scale bars, 1 s, 20 μm. Traces are reported in the lower panel. Scale bars, 1 s, 30 a.u. (b) Quantitative data for myocytes from male mice at 3–6 months (Young, n=120 cells from 4 hearts) and 27 months (Old, n=131 cells from 5 hearts) are shown as mean±s.e.m. and scatter plots. Caff, caffeine. *P<0.001 versus Paced (Wilcoxon Signed Rank Test); †P<0.01 versus Young (Mann–Whitney rank sum Test). (c) Quantitative data for the caffeine-induced Ca2+ transient decay obtained from a subset of cells represented in b (Young, n=90 cells from 4 hearts; Old, n=86 cells from 5 hearts) are shown as mean±s.e.m. and scatter plots. *P<0.05 versus Tyr (Mann–Whitney rank sum test).
INaL and modulation of myocyte mechanics and Ca2+ transients
The prolonged APD explains only in part the defective properties of Ca2+ transients in old myocytes. Additional factors potentially contributing to the alterations in Ca2+ transient decay were considered. Enhanced Na+ influx via INaL may increase intracellular Na+ load in old cardiomyocytes34,35,36,37, a condition inhibiting diastolic Ca2+ extrusion via NCX and affecting Ca2+ transient decay26,37,38. To test this possibility, INaL was inhibited in field-stimulated myocytes while monitoring Ca2+ transients and cell shortening. Consistent with the shortening of the duration of the AP, this intervention decreased the amplitude and time to peak Ca2+ transients and cell twitching. However, the kinetics of Ca2+ decay and myocyte relengthening were reduced in old cells, in contrast with the changes observed with AP-clamp tests (Fig. 7a,b and Supplementary Fig. 23). Therefore, 4-aminopyridine (4-AP) or anemonia toxin-II (ATX-II) were employed to prolong the duration of the AP (Supplementary Fig. 24) by selectively inhibiting Kv currents11,22,24 or by enhancing INaL (refs 29, 39), respectively. In both cases, the prolongation of the AP increased the amplitude of Ca2+ transients and delayed time to peak of Ca2+. However, 4-AP led to a faster Ca2+ transient decay, whereas the enhanced INaL with ATX-II failed to shorten the kinetics of Ca2+ decay (Fig. 7c,d). Collectively, these findings suggest that enhanced Na+ influx opposes the faster Ca2+ decay associated with increases in APD.
(a) Quantitative data for Ca2+ transients in myocytes from male mice at 30–33 months, before (Tyr) and after exposure to 10 μM ranolazine (Ran) (n=19 cells from 5 hearts); data are shown as mean±s.e.m. and scatter plots. *P<0.05 versus Tyr (paired t-test). (b) Quantitative data for unloaded cell shortening properties in myocytes from male mice at 30–33 months, before (Tyr) and after exposure to 10 μM ranolazine (Ran) (n=29 cells from 4 hearts); data are shown as mean±s.e.m. and scatter plots. *P<0.05 versus Tyr (paired t-test). (c) Quantitative data for Ca2+ transients in myocytes from male mice at 3–6 months, before (Tyr) and after exposure to 0.5 mM 4-aminopyridine (4-AP) (n=10 cells from 2 hearts); data are shown as mean±s.e.m. and scatter plots. *P<0.01 versus Tyr (paired t-test and Wilcoxon Signed Rank Test); NP, non-parametric analysis. (d) Quantitative data for Ca2+ transients in myocytes from mice at 3–9 months, before (Tyr) and after exposure to 1 nM anemonia toxin-II (ATX-II) (n=14 cells from 4 hearts); data are shown as mean±s.e.m. and scatter plots. *P<0.001 versus Tyr (paired t-test and Wilcoxon Signed Rank Test); NP, non-parametric analysis.
INaL, myocardial and cardiac function
To determine whether alterations in INaL and its effects on senescent cardiomyocytes were equally operative at the level of the myocardium, LV papillary muscles from young, 3–6 months, and old, 30–33 months, hearts were studied in an isometric system. Developed tension was similar in both muscles, but twitch kinetics, measured as the time of relaxation from the beginning of contraction, was prolonged with aging (Fig. 8a,b). Inhibition of INaL with ranolazine or mexiletine reduced developed force and accelerated relaxation times, which were more apparent in old muscles (Fig. 8c,d and Supplementary Fig. 25). In addition, to define the adaptation of papillary muscles to the protracted electrical recovery and enhanced Na+ entry, 4-AP and ATX-II were employed. 4-AP had a positive inotropic effect, which was coupled with faster twitches (Fig. 8e); however, the enhanced INaL with ATX-II increased developed force but failed to accelerate myocardial contractile dynamics (Fig. 8f).
(a) Isometric contraction for young and old muscles. Scale bars, 500 ms, 0.5 mN mm−2. Traces are superimposed in the inset. Scale bars, 50 ms, 0.5 mN mm−2 (b) Properties of papillary muscles from male mice at 3–6 (Young, n=41) and 30–33 months (Old, n=24) are shown as mean±s.e.m. and scatter plots. *P<0.001 versus Young (Student’s t-test); NP, non-parametric analysis. (c) Contraction for an old muscle prior (black traces) and following ranolazine exposure (Ran, red traces). Scale bars, 500 ms, 0.6 mN mm−2. Traces are superimposed in the inset. Scale bars, 50 ms, 0.6 mN mm−2. (d) Data for muscles of male mice at 3–5 (Young, n=11) and 30–33 months (Old, n=14) before and after exposure to 10 μM ranolazine are mean±s.e.m. and scatter plots. *P<0.001 versus KHB (Wilcoxon signed rank test); NP, non-parametric analysis. (e) Data for muscles of male mice at 3–6 months (n=9) before and after exposure to 1 mM 4-AP are mean±s.e.m. and scatter plots. *P≤0.001 versus KHB (paired t-test). (f) Data for muscles of mice at 3–9 months (n=9) before and after exposure to 10 nM ATX-II are mean±s.e.m. and scatter plots. *P<0.05 versus KHB (paired t-test). (g) Diastolic length–tension relations for twitching muscles stimulated at 6 Hz in the absence or presence of mexiletine (Mex). Muscles were obtained from male mice at 3 (Young, n=22; Young-Mex, n=6) and 30–33 months (Old, n=8; Old-Mex, n=8); data are mean±s.e.m. Fitting: second-order polynomial function (see Supplementary Table 3). *P<0.05 between selected fittings (one-way ANOVA with Bonferroni’s post hoc test). (h) Diastolic length–tension relations of muscles from male mice at 3 months in the absence (Young, data as in g and presence of the INaL enhancer ATX-II (n=10); data are shown as mean±s.e.m. and fitted with a second order polynomial function. *P<0.0001 between fittings (Student’s t-test). Parameters are reported in Supplementary Table 3.
Subsequently, the influence of Na+ influx and altered twitch kinetics on diastolic tension of the myocardium was evaluated. The passive length–tension relationship was measured by progressively stretching papillary muscles stimulated at 6 Hz, from near slack length (L0) to the length at which maximal force (Lmax) was developed. Diastolic tension was higher in the senescent myocardium and inhibition of Na+ influx shifted the relation to lower passive tension (Fig. 8g). In contrast, enhancement of Na+ entry with ATX-II in young muscles shifted the diastolic length–tension curve to higher values (Fig. 8h).
To determine whether the improved muscle kinetics following inhibition of INaL had functional consequences on cardiac performance in vivo, LV haemodynamics were assessed in young and old mice prior to and after inhibition of INaL, using an i.v. infusion protocol (Supplementary Fig. 26a). Blockade of the INaL with ranolazine in old mice led to faster contractile dynamics as documented by a shortened systolic duration and time constant of pressure decay, and increased −dP/dt (Fig. 9a and Supplementary Fig. 26b). These effects were attenuated in young animals. Importantly, in vivo infusion of ranolazine reduced the slope of the LVEDPV relationship in aged mice (Fig. 9b,c). Thus, INaL modulates the contractile and diastolic properties of cardiomyocytes, LV myocardium and whole heart.
(a) Quantitative data for LV haemodynamic parameters obtained in male mice at 5–6 months (Young) and at 29–33 months (Old) at baseline (Base) and ∼10 min following bolus infusion of ranolazine (2.5–5 mg per kg body weight, i.v.) (Young, n=7; Old n=6). Data are shown as mean±s.e.m. and scatter plots. *P<0.05 versus Base (paired t-test). Additional haemodynamic parameters and control experiments with saline infusion are reported in Supplementary Fig. 26. (b) PV loops during inferior vena cava occlusion protocol obtained in two male mice at 27 months at baseline and after bolus infusion of ranolazine (2.5 mg per kg body weight, i.v.). Scale bars, 3 μl, 20 mm Hg. (c) Quantitative data for LVEDPV slope obtained in male mice at 27 months at baseline (Base) and following bolus infusion of ranolazine (n=4). Data are shown as mean±s.e.m. and scatter plots. *P<0.05 versus Base (paired t-test).
Molecular determinants of myocyte aging
The gene expression for K+ and Na+ channels was evaluated in isolated preparations of young and old cardiomyocytes. The transcripts of Kcnd2, Kcnd3 and Kcnb1 genes, which encode, respectively, the K+ channel subunits Kv4.2, Kv4.3 and Kv2.1 were not affected by aging (Supplementary Fig. 27a). In contrast, the mRNAs of the Kcna4, Kcna5 and Kcnip2 genes, which encode the Kv1.4 and Kv1.5 channel subunits, and the Kv channel-interacting protein 2 or KChIP2, were decreased in old myocytes (Fig. 10a). These changes at the mRNA level are consistent with the attenuated density of Kv currents measured electrophysiologically (see Fig. 3b).
(a) Quantitative data for the expression of genes related to channels mediating Ito (Kcna4, Kcnip2) and IK,slow1 (Kcna5) in myocytes from mice at 3–4 months (Young, n=5–8) and 27–33 months (Old, n=5–8) are shown as mean±s.e.m. and scatter plots. RQ, relative quantity with respect to β-2-microglobulin; *P<0.05 versus Young (Student’s t-test and Mann–Whitney rank sum test); NP, non-parametric analysis. (b) Expression of the Na+ channel protein subunit Nav1.5 by western blotting in the LV myocardium of mice at 3–5 months (Young, n=6) and 28 months (Old, n=6). GAPDH is the loading condition. Quantitative data are reported as mean±s.e.m. and scatter plots. (c) Expression of Na+ channel β1 subunit protein by western blotting in the LV myocardium of mice at 3–4 months (Young) and 30–31 months (Old). β1 levels in the brains of Scn1b null (KO) and wild-type (WT) mice were used as negative and positive controls, respectively. A total of 150 μg of LV protein lysate per lane and 25 μg of brain membrane protein per lane were loaded.
The mRNA levels of the Scn5a, Scn1b, Scn2b and Scn4b genes, which encode, respectively, the Na+ channel α-subunit Nav1.5 and the Na+ channel subunits β1, β2 and β4 did not differ significantly in young and old myocytes (Supplementary Fig. 27b). Transcripts of the isoforms of the Na+ channel α-subunit, typically found in the central nervous system (Nav1.1, Nav1.3, Nav1.6) and skeletal muscle (Nav1.4) were barely detectable or were comparable in young and old cardiomyocytes (Supplementary Fig. 27b). Similarly, the expression of Nav1.5 protein was preserved with age, while the levels of the β1 subunit, which modulates Na+ channel gating40,41,42, were barely detectable, with no measurable differences between young and old samples (Fig. 10b,c and Supplementary Fig. 27c). These findings support the possibility that post-translational modifications or alterations of the Na+ channel multiprotein complex may have contributed to the alteration of INaL with age18,25,43,44,45,46.
Quantitative reverse transcription–PCR (qRT–PCR) was then employed to define the expression of Ca2+ handling molecules in cardiomyocytes. Transcripts of the Cacna1, Slc8a1, Ryr2 and Pln genes, which encode, respectively, the Ca2+ channel α-subunit Cav1.2, NCX, ryanodine receptor type-2 and phospholamban, were comparable in young and old myocytes. However, the mRNA of the Atpa2a2 gene, encoding SERCA2a, was reduced in old cardiomyocytes (Supplementary Fig. 28). However, this change was no longer present at the protein level (Supplementary Fig. 29). Thus, the Ca2+ handling machinery is largely preserved in aging cardiomyocytes, strengthening the notion that defects in the electrical properties of these cells contribute in part to the abnormalities in Ca2+ cycling identified in the old heart.
Discussion
The results of the present study document that myocardial aging is characterized by a slower electrical repolarization and protracted kinetics of contraction, which contribute to the alterations in the contractile and relaxation function of the old heart. The mechanisms involved in the modifications of ventricular compliance have been predominantly ascribed to collagen accumulation in the myocardial interstitium1,4,6,47. However, our findings indicate that physiological aging is not associated with diffuse interstitial fibrosis and/or multiple foci of tissue scarring in the myocardium, strengthening the critical role that the remodelled electromechanical characteristics of myocytes have in the diastolic and systolic properties of the aged heart.
The animal model employed here reiterates some of the features of human myocardial aging1,2. In the absence of systemic hypertension, 24–25-month-old mice show impaired diastolic function; EF and LV end-diastolic volume are preserved, but an increase in LV weight occurs with age. In contrast, in older animals at 30–35 months of age, deterioration of systolic performance, cavitary dilation and myocardial hypertrophy become noticeable, and these changes appear to be independent from the alterations in the diastolic indices observed at earlier time points. The elevated end-diastolic pressure and the perturbations in LV filling found at ∼2 years of age are coupled with protracted electrical recovery, corroborating recent observations in patients where the long QT interval has been linked to diastolic dysfunction48. The prolonged QT interval may be caused by lengthening of the AP and/or by the electrical heterogeneity of the ventricular myocardium, characterized by transmural voltage gradients and dispersion of repolarization49. However, our data, in which local epicardial recording was combined with measurements in isolated myocytes, strongly suggest that the shape of the AP is a major determinant of the abnormal electrical recovery of the senescent heart.
Changes in autonomic regulation occur with age15,16, a factor that may have attenuated the cardiac contractile reserve of the old heart. EF is reduced at 30 months and complete autonomic blockade blunts this difference in conscious animals. However, it has no effects on the protracted electrical repolarization and impaired diastolic indices of ventricular haemodynamics collected in anaesthetized mice. Caution has to be exercised when data obtained in conscious and anaesthetized animals are compared, although both the in vivo and ex vivo results tend to suggest that defects in neurohumural regulation have modest consequences on the electrical and passive mechanical properties of the senescent myocardium.
Our findings support the notion that the protracted electrical recovery with aging contributes to the alterations in myocyte relengthening and diastolic stiffness of the myocardium. Reduction in the rapidly activating Kv currents and increase of INaL are the ionic substrates responsible for the prolongation of the AP in aging cells. But whether gene regulatory networks and/or the activity of post-translational modulators are modified in aged cardiomyocytes, impacting on K+ and Na+ channel mutiprotein complexes, remains to be determined18,25,43,44,45,46,50.
The expression of Kv channel subunits is decreased in the post-infarcted heart in rodents and this condition is associated with reduced outward K+ current, prolonged AP and increased Ca2+ transient amplitude23. Although limited information is available in diseased mouse models with altered Na+ channel levels, genetic deletion of Scn1b in mouse cardiomyocyte results in longer APD, increased Ca2+ transients and slower Ca2+ decay; these defects, however, are reversed by low dose of TTX51. These observations and the current data in aging mice are consistent with the critical role that Kv and Na+ regulatory channel subunits have in modulating the electrical and mechanical properties of cardiomyocytes.
Reduced outward Kv currents and enhanced INaL contribute to the electrical recovery of old myocytes by altering, respectively, the early and late repolarization phase of the AP. Inhibition of INaL in old myocytes critically shortens APD90 to values found in young cells. However, this pharmacological intervention has minimal effects on the initial repolarization phase of the AP. Similarly, reduction of INaL in vivo reduces the duration of the QT interval in old mice restoring partly the electrical recovery of the myocardium. The negligible impact of ranolazine and mexiletine on the QT interval in young mice may reflect the attenuated density of INaL found in isolated cardiomyocytes.
In the failing heart, SERCA activity is attenuated and results in abnormal Ca2+ handling, which is centrally involved in the decline of cardiomyocyte performance52. However, SERCA protein is preserved in aging myocytes, suggesting that the cellular mechanisms implicated in the aging myopathy may be distinct from those typically found in chronic heart failure52. Although a potential role of SERCA with defective Ca2+ re-uptake has to be considered in the development of the decompensated aging heart, the increase in INaL, together with the modifications in the electrical properties of cardiomyocytes, has to be viewed as an important variable of the progressive alterations in contractile kinetics and deterioration in diastolic and systolic properties of the senescent muscle.
Our results indicate that enhanced INaL mediates protracted repolarization of the AP and Na+ influx, potentiating the amplitude of Ca2+ transients and exerting a positive inotropic effect. Similarly, the decrease in Kv currents favours the prolongation of the AP promoting Ca2+ influx via L-type channels and reduced Ca2+ efflux via NCX8,9,10,11. Yet, the amplitude of Ca2+ transients and cell contractility are maintained with aging, in spite of prolonged APD, suggesting that the cellular defects in old myocytes condition the efficiency of the positive APD-inotropic relationship, which may be in part attributed to the reduced SR Ca2+ load. Thus, the changes in INaL and Kv currents may be interpreted as necessary responses aiming at the preservation of systolic function at the expense of a loss in cellular and ventricular compliance.
The prolonged AP in aging myocytes correlates with the delayed peak of Ca2+ transients and cell shortening, indicating that the slower repolarization phase sustains the Ca2+-induced Ca2+ release mechanism, increasing the translocation of Ca2+ from the SR to the cytoplasm. The ionic events underlying the positive inotropic action of enhanced INaL and the protracted AP may comprise an increased Na+ influx and enhanced [Na+]i (ref. 41). The higher intracellular Na+ load is balanced by an increase in intracellular Ca2+ since the elevated Na+ attenuates the extrusion of Ca2+ via the NCX9,37,41. This form of inotropic support of myocyte contractility appears to be implicated in the complex adaptation of the myocardium to chronological aging. INaL may have a dual effect: it prolongs the AP and it influences indirectly the Ca2+ load. These variables affect, on the one hand, systolic Ca2+ release and contraction and, on the other, diastolic Ca2+ clearance and relaxation.
The central role of Na+ influx and enhanced Na+ load on the protracted Ca2+ transient decay in aged myocytes is corroborated by the findings in which the AP was prolonged by inhibition of Kv currents or enhancement of INaL. Both approaches led to an increase in Ca2+ transient amplitude, but differentially modulated the timing of Ca2+ decay. Similarly, these interventions had a positive inotropic action on papillary muscles, but differently affected contractile dynamics: twitch kinetics were shortened with 4-AP, but were not altered with ATX-II. Importantly, when [Na+]i was stabilized using cells dialysis and patch-clamp, differences in Ca2+ transient decay between young and old cells were abrogated. The faster caffeine-induced Ca2+ transient decay observed in old myocytes is suggestive of an increased NCX activity, which may reflect an adaptation to the altered Na+ load.
Inhibition of INaL in old myocytes shortens the AP and corrects the kinetics of Ca2+ transients, cell contraction and relaxation. Similarly, the repolarization and compliance of the senescent myocardium are restored, but muscle developed tension is attenuated. INaL modulates both the systolic and diastolic properties of the myocardium. However, in old mice, inhibition of INaL in vivo ameliorated diastolic indices and shortened systolic duration without interfering with developed pressure. Whether the blunted effects on developed pressure are due to compensatory adaptations inherent in the protocol employed requires further analysis. Thus, chronological age affects the electrical and molecular identity of cardiomyocytes, which are critical determinants of the pathological manifestations of the old heart.
Methods
Animals and in vivo studies
Male and female C57Bl/6 mice from 3 to 35 months of age were used in this study. When male or female mice were employed, gender is specified in the figure legend. Animals were maintained in accordance with the Guide for Care and Use of Laboratory Animals; animal experiments were approved by the local animal care committee (IACUC). When needed, isoflurane (1–1.5%, inhalation) was employed as a methodology of anaesthesia.
Scn1b WT and null littermate mice were generated and maintained in accordance with the guidelines of the University of Michigan Committee on the Use and Care of Animals. Mice were bred from Scn1b heterozygous animals that had been repeatedly backcrossed to C57Bl/6 mice for at least 18 generations, creating a congenic strain. Mice at postnatal day17 were anaesthetized and brains were removed for membrane preparations and subsequent western blot analysis, as previously done51.
In the attempt to define the effects of aging on LV function, anatomy and structure in mice, several assays were employed. The broad experimental approach was designed to obtain detailed information in vivo and to overcome limitations intrinsic to various experimental protocols and acquisition systems.
Blood pressure in conscious mice was analysed with a computerized, non-invasive tail-cuff plethysmography system (BP 2000 Blood Pressure Analysis System, Visitech Systems). For each mouse, 10 inflation–deflation cycles were used initially to set amplifier and instrument controls. Subsequently, systolic blood pressure was calculated by averaging readings from 40 cycles.
ECG was performed in conscious mice using a Visualsonics Vevo 2100 System equipped with a MS550D high frequency (22–55 MHz) linear transducer. By this approach, long and short axis views of the LV were analysed32. Diastolic function was assessed using pulsed-wave Doppler imaging of the transmitral filling pattern in the apical four-chamber view of the heart. Early transmitral filling wave (E-wave) and late filling wave due to atrial contraction (A-wave) were obtained. Isovolumic relaxation time was calculated as the time from closure of the aortic valve to the initiation of the E-wave, and isovolumic contraction time was calculated as the time from closure of the mitral valve to opening of the aortic valve. The deceleration time of the E-wave was determined by measuring the time needed for the down-slope of the peak of the E-wave to reach baseline17.
For high-frequency speckle-tracking imaging17, mice were anaesthetized and B-mode loops were recorded in the parasternal long- and short-axis views, while continuous ECG monitoring was obtained via limb electrodes. Three consecutive cardiac cycles were selected and analysed with Vevostrain software (Visualsonics). Strain measures were averaged over the cardiac cycles resulting in curvilinear strain and strain rates. In both the long- and short-axis views, the LV myocardium was divided into six standard anatomic segments, which were averaged for global speckle-tracking-based strain analysis throughout the cardiac cycle. Images collected with suboptimal physiological parameters or inadequate visualization of the endocardial border were excluded.
MRI was performed with a 7 T Pharmascan (Bruker) system equipped with a mouse cardiac coil in birdcage design (Rapid Biomedical) to obtain cine volumetric short axis13. To reduce motion artifacts and heart rate, animals were anesthetized with isoflurane by nose cone and placed into the coil in prone position. Electrodes were attached to the front left paw and right leg for electocardiographic gating and monitoring of vital signs using a gradient echo sequence (echo time 2.6 ms, 16 frames per RR interval; flip angle 30°; in-plane resolution 200 × 200 μm; slice thickness 1 mm). Animals were kept warm by blowing hot air into the magnet. The heat flow and the anaesthesia level were manually adjusted to maintain heart rate. Anatomical and functional parameters were quantitated from six to eight cine short axis imaging slices covering the LV.
ECGs were recorded under isoflurane anaesthesia by inserting needle electrodes s.c. into the mouse limbs (lead II). Electrical signals were amplified (Animal Bio Amp, ADInstruments), digitized using a 4 kHz A/D converter (MPVS-400, Millar Instruments) and recorded using LabChart software (ADInstruments), with low and high-pass filtering at 100 Hz and 3 Hz, respectively. Surface ECG intervals were measured using LabChart7 or LabChart8. Spontaneous cycle length was determined by averaging 10 consecutive RR intervals. PR and QT intervals were measured by determining the earliest onset and latest offset of atrial and ventricular deflections from the averaged cycles (Supplementary Fig. 30)53.
To record ECGs in freely roaming, unanaesthetized animals, telemetric biopotential transmitters (ETA-F10, Data Science International) were implanted surgically in anaesthetized mice54. Surgery was performed in anaesthetized mice. The peritoneum was exposed with a midline incision, the implant was secured within the peritoneal cavity, and leads were implanted s.c. corresponding to position II. Subsequently, signals were transmitted from implants to RPC-1 receivers and Data Exchange Matrix (Data Science International), and stored in a computer. The telemetric ECG was analysed during normal activity and following pharmacological treatments. All recordings were digitized at 2 kHz and analysed off-line with Ponemah 5.10 software.
LV haemodynamics and PV loops were obtained in anaesthetized mice (isoflurane, 1.5%) in the closed chest preparation with a MPVS-400 system for small animals equipped with a PVR-1045 catheter32,55,56,57,58. The mouse was intubated and ventilated (MiniVent Type 845, Hugo Sachs Elektronik-Harvard Apparatus) with isoflurane anaesthesia and warmed with a heat lamp; the right carotid artery was then exposed and the pressure transducer was inserted and advanced in the LV cavity. Data were acquired with Chart 5 or LabChart8 software. Baseline PV loops and loops following inferior vena cava or aortic arch occlusions were collected (Supplementary Fig. 31) to compute the slope of end-diastolic PV relations, which is an indicator of LV stiffness57. During data collection, ventilation was interrupted to acquire signals without motion artifacts. Inferior vena cava occlusion was achieved in the closed chest preparation by compression of the inferior vena cava accessed immediately below the diaphragm via a small abdominal incision, with a cotton tip applicator58. For aortic arch occlusion, a thoracotomy through the sternoclavicular articulation provided exposure of the aortic arch allowing its transient constriction with a bulldog clamp59. For calibration of PVR-1045 catheter and evaluation of LV blood volume, manufacturer’s instructions were followed. Cuvette calibration with fresh heparinized, warm blood and in vivo bolus infusion of hypertonic saline solution were performed to compute slope and intercept and to assess parallel conductance. Analysis was performed using LabChart and PVAN (Millar) software. Systolic duration was computed by LabChart as the elapsed time between the start of the cycle at LV end-diastolic pressure and the time of maximal −dP/dt.
For combined autonomic blockade, atropine (0.5 mg per kg body weight, i.p., Hospira) plus propranolol (1 mg per kg body weight, i.p., Fluka) were administered to animals14,60,61. In vivo blockade of the late Na+ current (INaL) was achieved with administration of ranolazine (30 mg per kg body weight, i.p. or 2.5–5 mg per kg body weight, i.v., Sigma)62 or mexiletine (5 mg per kg body weight, i.p., Sigma)63. Compounds were dissolved in United States Pharmacopeia (USP) saline solution. Ranolazine bolus was delivered via left jugular vein using a syringe pump (PHD Ultra, Harvard Apparatus).
Ex vivo properties of the mouse heart
To assess ex vivo electrical properties hearts were perfused through the aorta in a Langendorff apparatus (Radnoti) at a constant pressure of 80 mm Hg with Krebs–Henseleit buffer (KHB; Sigma), containing in mM: 118 NaCl, 4.7 KCl, 11 glucose, 1.2 MgSO4, 1.2 KH2PO4, 1.8 CaCl2 and 25 NaHCO3, gassed with 95% O2 and 5% CO2 (pH 7.4) at 37 °C (refs 12, 64). The temperature was maintained by immersing the heart in a water-heated glassware reservoir (Radnoti), containing preheated KHB. Hearts were stimulated with a 2-ms square pulse at 1.5-fold its threshold level (four channels stimulator, BMS 414, Crescent Electronics), using a mini-coaxial electrode (Harvard Apparatus). A two-lead mini ECG system (Harvard Apparatus), in which electrodes were placed on the right atrium and apex of the heart, was used to obtain pseudo-ECG. MAPs were recorded using a micro MAP-Tip electrode (Harvard Apparatus) positioned on the LV free wall12,64. ECG and MAP signals were amplified (Animal Bio Amp), digitized by a 4 kHz A/D converter (PowerLab 8/30, ADInstruments) and recorded with LabChart software, with low and high-pass filtering at 1 kHz and 0.3 Hz, respectively. A protocol of PES was introduced to assess the propensity of the mouse heart to develop ventricular arrhythmia. PES consisted of a train of pacing stimuli (S1) applied at 125 ms cycle length, with an extra stimulus (S2) inserted every eight beats. The S1–S2 interval was progressively reduced until the S2 stimulus either failed to generate an AP or induced arrhythmic events. The appearance of ventricular tachycardia (three or more consecutive ectopic beats characterized by atria-ventricular dissociation) and/or ventricular fibrillation was established12,64. Data were analysed with LabChart software.
Myocyte isolation
Hearts were excised and LV myocytes were enzymatically dissociated using a methodology that was previously employed11,12,32,55,56. The heart was connected to a plastic cannula for retrograde perfusion through the aorta in a Langendorff system (Radnoti) at 37 °C. Perfusate consisted of a Ca2+-free solution gassed with 85% O2 and 15% N2. After 5 min, 0.1 mM CaCl2, 274 U ml−1 collagenase (type-2, Worthington Biochemical Corp) and 0.57 U ml−1 protease (Type XIV, Sigma) were added to the solution, which contained in mM: NaCl 126, KCl 4.4, MgCl2 5, HEPES 5, Glucose 22, Taurine 20, Creatine 5, Na Pyruvate 5 and NaH2PO4 5 (pH 7.4). At completion of digestion, atria and right ventricle were dissected and LV myocardium was cut in small pieces and these fragments were shaken in re-suspension solution and filtered using a 200 μm nylon mesh (Spectrum Labs). Aliquots of cell suspensions were centrifuged for 5 min at 100g (Eppendorf 5702R) at 4 °C and frozen for biochemical assays and fixed in paraformaldehyde (4%, Electron Microscopy Sciences). For electrophysiological and mechanical studies, only rod-shaped myocytes exhibiting cross striations, and showing no spontaneous contractions or contractures were selected; cells were used within 8 h following enzymatic digestion.
Myocyte volume
LV myocytes fixed in paraformaldehyde were stained with the nuclear dye Hoechst (10 μM, Sigma). Images were collected with a fluorescent inverted microscope (Olympus IX71) equipped with a CCD camera (Hamamatsu ORCA-R2). Cell length and area were evaluated using ImageJ software. Myocyte volume was then computed assuming an elliptical cross-section, in which the major axis corresponds to the average cell width, and the minor axis was computed after establishing the ratio between the major and minor axis, using three-dimensional optical section reconstruction by two-photon microscopy (BX51WI Olympus microscope coupled with a Bio-Rad Radiance 2100MP system) and image analysis (ImageJ). Three-dimensional reconstructions were obtained using second-harmonic generation signal of sarcomeric structures65.
Histological analysis
Mouse hearts were fixed in phosphate-buffered formalin (10%, Sigma) and embedded in paraffin. Sections 4-μm thick of the LV were trichrome-stained for detection of connective tissue (Gomori’s One Step Trichrome Method, Poly Scientific R&D Corp.) following manufacturer’s instructions. Images were acquired using an upright microscope (Olympus BX60) with × 20 objective equipped with a colour camera (Olympus DP73). Interstitial fibrosis was quantified with respect to total tissue area using ImageJ software.
Cell shortening and Ca2+ transients
Isolated LV myocytes were placed in a bath on the stage of an Axiovert (Zeiss), IX71 and BH-2 (Olympus) microscopes for contractility and Ca2+ transients11,12,32,55,56. Experiments were conducted at room temperature. Cells were bathed continuously with a Tyrode solution containing (in mM) NaCl 140, KCl 5.4, MgCl2 1, HEPES 5, Glucose 5.5 and CaCl2 1.0 (pH 7.4, adjusted with NaOH). Measurements were collected in field-stimulated (S88 and SD9 Grass stimulators) cells by IonOptix fluorescence and contractility systems (IonOptix), or by video edge detection (VED-205, Crescent Electronics; PowerLab 8/35, ADInstruments). Contractions were elicited by rectangular depolarizing pulses, 2 ms in duration and 1.5 times threshold in intensity, with platinum electrodes. Ca2+ transients were measured by epifluorescence after loading the myocytes with 10 μM Fluo-3 AM or 2 μM Fluo-4 AM (Invitrogen). Excitation length was 480 nm with emission collected at 535 nm using a × 40 objective. Fluo signals were expressed as normalized fluorescence (F/F0), where F0 is the diastolic fluorescent level subtracted by the background signal measured in the region adjacent to the cell12.
Unless otherwise specified, the late Na+ current (INaL) was blocked with a low dose of ranolazine (10 μM, Sigma) or mexiletine (10 μM, Sigma), or enhanced with anemonia toxin-II (1 nM, Sigma)25,26,28,29,39,66,67,68. Rapidly activating Kv outward currents were blocked with 0.5 mM 4-AP (Sigma)11,22,24.
Caffeine-induced Ca2+ transients
To evaluate SR Ca2+ load in field-stimulated myocytes, a 2 s caffeine (20 mM, Sigma) spritz was delivered using glass pipettes and a microinjector (FemtoJet, Eppendorf AG, or Pico-liter Injector, Warner Instruments), and Ca2+ transients recorded by epifluorescence microscope and IonOptix system, or two-photon microscope11,12,32,55. For two-photon imaging, myocytes were loaded with 5 μM Fluo-4 AM and placed on the stage of an upright microscope (BX51WI Olympus microscope coupled with a Bio-Rad Radiance 2100MP system). Cells were bathed with Tyrode solution and field stimulated using platinum electrodes. Fluo-4 was excited at 920 nm wavelength with mode-locked Ti/sapphire femtosecond laser (Tsunami, Spectra-Physics), and the emission signal was collected at 535 nm. Images were acquired in line scan mode with the myocyte oriented in the long axis, at 6 ms sampling rate12,55. Fluo signals were expressed as normalized fluorescence (F/F0), where F0 is the diastolic fluorescent level subtracted by the background signal measured in the region adjacent to the cell12. Due to potential leak of caffeine from the glass pipette and undesired facilitation of Ca2+ release, cells displaying paced-induced Ca2+ transients larger than 5 F/F0 (corresponding to values above average+2·s.d.) were excluded from the analysis.
Na+/Ca2+ exchange (NCX) activity was evaluated by the decay of the caffeine-induced Ca2+ transient, which was fitted with a mono-exponential function33,69. Cells presenting prolonged Ca2+ transient decay (>4.8 s, corresponding to values >average+2·s.d.) and/or poor fitting (R2<0.96) were excluded from the study, without affecting statistical results.
Whole-cell patch-clamp studies
Isolated LV myocytes were placed in a bath on the stage of an IX51, IX53, and IX71 microscopes for patch-clamp measurements11,12,32,55,56,66. Experiments were conducted at room temperature. Data were acquired by means of the whole-cell patch-clamp technique in voltage- and current-clamp modes using Multiclamp 700A and 700B, and Axoclamp 900A amplifiers (Molecular Devices). Electrical signals were digitized using 250 kHz 16-bit resolution A/D converters (Digidata 1322, 1440A, and 1550, Molecular Devices) and recorded using pCLAMP 9.0 and 10 software (Molecular Devices) with low-pass filtering at 2 kHz. Membrane capacitance (Cm) was measured in voltage-clamp mode using a 5 mV voltage step and pCLAMP software algorithm. For each cell, current density was obtained by dividing transmembrane currents recorded in voltage-clamp by the measured Cm, providing a normalization of ionic currents with respect to cell size. Current density was expressed in pA pF−1 (refs 11, 12, 32, 55, 56). Pipettes were pulled by means of a vertical (PB-7, Narishige), or horizontal (P-1000, Sutter Instrument) glass microelectrode pullers; when filled with intracellular solution pipettes had a resistance of 1–2 MΩ.
Action potential measurements
For AP measurements, current-clamp mode was employed11,12,66. Cells were stimulated with current pulses 1.5 times threshold. Myocytes were bathed with Tyrode solution. The composition of the pipette solution was (in mM): NaCl 10, KCl 113, MgCl2 0.5, K2-ATP 5, glucose 5.5, HEPES 10, EGTA 10 and CaCl2 1 (pH 7.2 with KOH). To test the effects of ion channels modulators on the electrical properties of myocytes, APs were continuously recorded for the same cell before and after exposure to agonist or inhibitors. The following compound were used in current-clamp experiments: ranolazine (10 μM), mexiletine (10 μM), anemonia toxin-II (1 nM)25,26,28,29,39,66,67,68 and 4-AP (0.5 mM)11,22,24.
Measurements of voltage-gated K+ currents
Voltage-gated outward K+ Kv currents were assessed in voltage-clamp mode with a previously reported protocol24, to dissect K+ currents with rapid activation and fast (Ito), intermediate (IK,slow1) and slow (IK,slow2) kinetics of inactivation, together with a sustained (non-inactivating) component (Iss)21,24. Cells were bathed with a modified Tyrode solution containing (in mM) NaCl 140, KCl 4, MgCl2 1, HEPES 10, Glucose 10, CaCl2 1.2 and 0.3 mM CdCl2 to block L-type Ca2+ current (pH 7.4, adjusted with NaOH)11,24. The composition of the pipette solution was (in mM): NaCl 5, KCl 20, K-aspartate 120, MgCl2 1, Mg-ATP 5, EGTA 10, HEPES 10 (pH 7.2 with KOH)24. Following membrane capacitance and series resistance compensation, Kv currents were activated using depolarizing voltage steps from −80 mV to +60 mV, 25 s in duration. Interpulse interval was 60 s. Ito, IK,slow1, IK,slow2 and Iss amplitude and time constants were obtained by fitting the current decay during the depolarizing step with a tri-exponential function using Clampfit 10 software24. Currents were normalized by Cm.
Measurements of voltage-gated Na+ currents
The late Na+ current (INaL) was measured in voltage-clamp mode25,67,68. Myocytes were bathed with a modified Tyrode solution in which KCl was replaced with CsCl and 4 μM nicardipine was added to block L-type Ca2+ current. Composition of the pipette solution was (in mM): NaCl 10, CsCl 113, MgCl2 0.5, Tris-ATP 5, glucose 5.5, HEPES 10, EGTA 5 and tetraethylammonium chloride 20 (pH 7.2 with CsOH). Myocytes were held at Vh of −120 mV, and INaL was elicited using 500-ms depolarizing pulses to −30 mV. The pulse was preceded by a 5 ms prepulse to +50 mV to optimize voltage control67. Interpulse interval was 5 s. INaL was measured as inward current decaying during the 500-ms depolarizing step to −30 mV, excluding the initial 20 ms of the depolarizing step. INaL was normalized by Cm. I–V relations were determined applying depolarizing steps 500 ms in duration from Vh −120 mV in 5 mV increments. The family of depolarizing steps was preceded by a 20-ms preconditioning step to −50 mV to activate the fast Na+ current. Interpulse interval was 5 s. INaL amplitude was measured as inward current decaying during the 500-ms depolarizing at various test potentials, excluding the initial 20 ms. INaL was normalized by Cm.
Equilibrium potential for Na+ at 25 °C was calculated by the Nernst equation:

At each potential tested (Vm), Na+ conductance (g) was calculated as

where INaL is the amplitude of the late Na+ current at Vm.
Conductance–voltage relations were plotted and fitted with Boltzmann function:

where gmax corresponds to maximal conductance, V1/2G the potential at which conductance is halfway between 0 and gmax and kG the slope of the conductance curve.
Values of normalized Na+ conductance (G=g · gmax−1) were plotted to obtain activation curves; half maximal activation potential (V1/2G) and slope of the activation curve (kG) were derived by fitting the data with Boltzmann equation:

A two-pulse protocol was utilized to assess the voltage dependence of steady-state inactivation of INaL. Prepulses were introduced to depolarize the cell to different membrane voltages ranging from −120 to +50 mV for 500 ms, in 10-mV increments. Each prepulse was followed by a single 500-ms test pulse, which depolarized the cell to −30 mV. Different prepulses were applied at 5-s intervals. Values of normalized Na+ conductance (G=g · gmax−1) were plotted with Vm relative to the preconditioning steps to determine steady-state inactivation curves, fitted with a Boltzmann equation, as indicated above (equation (4)).
In addition, INaL was assessed as TTX-sensitive current using depolarizing steps to −40 mV, 520 ms in duration from holding potential of −80 mV. The protocol was recorded for the same cell in Tyrode solution and following perfusion with 10 μM TTX (Sigma, Enzo Life Sciences). Scout depolarizations 10 mV in increments from Vh −80 mV and 5-s interpulse interval were applied every 1–2 min to evaluate the effects of TTX and experimental conditions. Difference current was obtained by subtracting traces recorded in TTX from those measured in Tyrode. The slowly decaying TTX-sensitive current was evaluated by quantifying the inward current decaying over the 520-ms depolarization, excluding the initial 20 ms of the depolarizing step. Currents were normalized by Cm. For these measurements, pipette solution was comparable to the one employed for AP measurements.
The fast Na+ current (INa) was measure in voltage-clamp mode70. Myocytes were bathed with a modified Tyrode solution of the following composition (in mM): NaCl 5, MgCl2 1, CaCl2 1, CdCl2 0.1, HEPES 20, glucose 11 and CsCl 132.5 (pH 7.4 with CsOH). The composition of the pipette solution was (in mM): NaCl 5, CsF 135, EGTA 10, Mg-ATP 5 and HEPES 5 (pH 7.2 with CsOH)70. I–V relations were determined applying depolarizing steps 200 ms in duration from Vh −120 mV in 5-mV increments. Interpulse interval was 3 s. INa amplitude was measured as the current difference between the inward peak current and the current at the end of the 200-ms step. INa was normalized by Cm.
A two-pulse protocol was utilized to assess the voltage dependence of steady-state inactivation of INa. Prepulses were introduced to depolarize the cell to different membrane voltages starting from −140 mV for 300 ms, in 5-mV increments. Each prepulse was followed by a single 30-ms test pulse, which depolarized the cell to −40 mV. Values of normalized Na+ conductance (G=g · gmax−1) were plotted with Vm relative to the preconditioning steps to compute the steady-state inactivation curves, which were fitted with a Boltzmann equation, as indicated above (equation (4)).
INa reactivation was studied using a two-pulse protocol with variable inter-pulse duration, ranging from 100 to 5ms, in decrements of 5ms. With each pulse, INa was activated by depolarizing cells from Vh −100 mV to −20 mV for 20ms. The double pulse protocol was repeated every 3s. For each interpulse duration (t), INa reactivation was calculated by dividing the amplitude of the current measured during the second pulse (I(t)) by the amplitude of the current measured during the first pulse (Imax). Values of INa reactivation were plotted to obtain reactivation curves and fitted with a mono-exponential function:

In voltage-clamp experiments in which Na+ currents were modulated pharmacologically with mexiletine (10 μM for quantification of the effects on INa; 30 μM for qualitative effects on INaL) or anemonia toxin-II (1 nM), depolarizing pulses between −60 mV and 0 mV, 10 mV in increments, 5-s interpulse interval, from Vh −70 mV or −90 mV (see legend to figure for details) were tested every 1–2 min to evaluate the effects of drugs and experimental conditions.
Measurements of L-type Ca2+ current
The L-type Ca2+ current (ICaL) was measured in voltage-clamp mode11,32,56. Myocytes were bathed with a modified Na+–K+-free Tyrode solution of the following composition (in mM): N-methyl-D-glucamine (NMDG) 140, CsCl 4, MgCl2 1, HEPES 5, glucose 5.5, CaCl2 1 and 4-AP 2 (pH 7.4 with CsOH). The composition of the pipette solution was (in mM): NMDG 10, CsCl 113, MgCl2 0.5, Tris-ATP 5, glucose 5.5, HEPES 10, EGTA 5 and TEA-Cl 20 (pH 7.2 with CsOH). ICaL I–V relation, conductance and activation properties were determined applying depolarizing steps 300 ms in duration from Vh −70 mV in 10-mV increments. ICaL amplitude was measured as the current difference between the peak inward current at the beginning of the step and the current at the end of the 300-ms pulse. ICaL was normalized by Cm.
Equilibrium potential for Ca2+ was defined by the intercept of the I–V relation to the zero-current axis. A linear fitting of the positive potential portion of the I–V relation was employed for this determination. At each potential tested (Vm), Ca2+ conductance (g) was calculated as

where ICaL is the amplitude of the L-type Ca2+ current at Vm.
Conductance–voltage relations were plotted and fitted with Boltzmann function (equation (3)). Values of normalized Ca2+ conductance (G=g · gmax−1) were plotted to obtain activation curves; half maximal activation potential (V1/2G) and slope of the activation curve (kG) were derived by fitting the data with Boltzmann equation (equation (4)).
A two-pulse protocol was utilized to assess the voltage dependence of steady-state inactivation of ICaL. Prepulses were introduced to depolarize the cell to different membrane voltages starting from −80 mV for 300 ms, in 10-mV increments. Each prepulse was followed by a single 300-ms test pulse, which depolarized the cell to 0 mV. Values of normalized Ca2+ conductance (G=g · gmax−1) were plotted with Vm relative to the preconditioning steps to determine steady-state inactivation curves, fitted with a Boltzmann equation, as indicated above (equation (4)).
Inactivation time course of ICaL was studied with a 300-ms depolarizing pulse at 0 mV (ref. 11). Traces were fitted between the inward peak and the end of the 300-ms pulse to 0 mV with a bi-exponential function66:

where Af and As are, respectively, the fraction of the fast- and slow-inactivating components, and C is the offset constant.
AP-clamp and Ca2+ transients
For AP-clamp experiments, Ca2+ levels in patch-clamped myocytes were assessed as fluorescence signal intensity of Fluo-loaded cells using a photomultiplier and a photon to voltage converter (IonOptix) connected to the patch-clamp A/D converter11,12. Cells were bathed in Tyrode solution and pipette solution was comparable to the one employed for AP measurements with the omission of EGTA and CaCl2.
Fluo signals were expressed as normalized fluorescence (F/F0), where F0 is the diastolic fluorescent level subtracted by the background signal measured in the region adjacent to the cell12. Duration of Ca2+ transient decay was evaluated at 10–90%, and obtained results from different cells were averaged and fitted with a mono-exponential function:

where FP and F0 are, respectively, the fluorescence relative to the peak and diastolic phase of the Ca2+ transient.
Isometric force in papillary muscles
Papillary muscles were dissected from the mouse LV and mounted in a horizontal tissue bath (Steiert, Hugo Sachs Elektronik-Harvard Apparatus) connected to a force transducer (F10, Harvard Apparatus)12. Muscles were superfused with KHB solution at 37 °C. The myocardium was stimulated by two platinum electrodes employing field stimulation (isolated stimulator output: frequency 6 Hz; pulse duration 2 ms; intensity 1.5-fold threshold; UISO, Hugo Sachs Elektronik-Harvard Apparatus). Each muscle was stretched to the length at which force of contraction was maximal. Muscle preparations were allowed to equilibrate for at least 30 min. Developed tension was measured isometrically with the force transducer attached to a Bridge Amp (ADInstruments) and a 4 kHz A/D converter (PowerLab 4/30, ADInstruments). Tension signal was recorded using LabChart 7 Pro software and analysed with LabChart8. Time to 50 and 90% relaxation are expressed with respect to the beginning of the twitch. Based on the premise that each muscle had a cylindrical shape, force measurements were normalized by the cross-sectional area of the muscle (mN mm−2). Digital images of sections were acquired using micro-zoom system microscopes (MVX10 and SZX16, Olympus) and a sCMOS camera (pco.edge) or a CCD camera (DP73), and analysed by ImageJ software12.
Passive diastolic tension was examined by progressively stretching muscles stimulated at 6 Hz, beginning from near slack length (L0) to the length at which the muscle developed maximal twitch tension (Lmax). Diastolic length–tension relations were plotted and fitted with a second-order polynomial equation:

where dT is diastolic tension and L is the length of the muscle.
The late Na+ current was blocked with ranolazine (10 μM, Sigma) or mexiletine (10 μM, Sigma), or enhanced with anemonia toxin-II (10 nM, Sigma)25,29,39,67. 4-AP (1 mM, Sigma) was employed to inhibit Kv currents and induce prolongation of myocyte AP11,22,24. Twitch properties were evaluated in young and old muscles at baseline and following superfusion with agonists or inhibitors. Muscles exposed to excessive stretching and displaying unstable twitches were excluded from the analysis. For evaluation of diastolic tension-length relations, muscles were superfused with KHB, mexiletine or anemonia toxin-II, and then progressively stretched under continuous electrical stimulation.
QRT–PCR
Total RNA was extracted from isolated mouse LV myocytes utilizing TRIZOL reagent (Invitrogen). Complementary DNA was obtained from 1 μg total RNA using MultiScribe reverse transcriptase kit (Applied Biosystems). Real-time RT–PCR was performed with primers designed using the Vector NTI Advance 11 (Invitrogen) software12,55,64. The sequences of primers are indicated in Supplementary Table 1. The StepOnePlus Real-Time PCR system (Applied Biosystems) was employed for quantitative RT–PCR. In each case, complementary DNA was combined with Power SYBR Green Master Mix (Applied Biosystems) in a 10-μl reaction. Cycling conditions were as follow: 95 °C for 10 min followed by 40 cycles of amplification (95 °C denaturation for 15 s, 60 °C annealing and extension for 1 min). The melting curve was then obtained. Ct values were normalized with respect to β-2-microglobulin (β2m). To avoid the influence of genomic contamination, forward and reverse primers for each gene were located in different exons. PCR products were run on 3% agarose/1 × TBE gel to confirm the specificity of the reaction. Total RNA extracted from mouse brain and skeletal muscle was employed as controls. For fibrotic markers18, RNA was extracted from LV tissue. Ct values were normalized with respect to hypoxanthine guanine phosphoribosyl transferase (Hprt)18.
Western blotting
Whole protein extracts from pellets of isolated LV myocytes or LV myocardium were prepared using RIPA buffer (Sigma), supplemented with a cocktail of protease inhibitors (Roche) and phosphatase inhibitors (Sigma). Equivalent of 15 μg of proteins were separated on SDS–PAGE, transferred onto polyvinylidene fluoride membrane, blocked with 5% BSA and exposed to the antibodies listed in Supplementary Table 2. Dilution is also indicated in Supplementary Table 2. Horseradish peroxidase-conjugated secondary antibodies (Cell Signaling Technology, 1:5,000 dilution) and Pierce ECL 2 western Blotting chemiluminescent substrate (Thermo Scientific) were utilized for signal detection12. Western blotting protocols with various antibodies were optimized before quantitative analysis. For molecular weight identification, Precision Plus Protein Kaleidoscope Prestained Protein Standards (Bio-Rad) was employed. Optical density of bands was measured using ImageJ and normalized by the expression of GAPDH.
For Na+ channel β1 subunit protein levels, samples were mechanically homogenized and sonicated in Tris-EGTA buffer (50 mM Tris, 10 mM EGTA) (for LV samples) or in RIPA buffer (Thermo) plus octyl β-D-glucopyranoside (Sigma-Aldrich) and protease inhibitors, with a brief low speed centrifuge spin (5 min, 5,000g) to remove debris and loaded with the addition of SDS-sample buffer. Approximately 150 μg of LV or myocyte lysate and 25 μg of brain membrane protein per lane were loaded. Brain membrane protein from wild-type and Scn1b null mice40 were employed as positive and negative controls, respectively. To obtain membrane proteins, a protocol previously used was employed here51. Brain tissue was immersed in 2 ml of Tris-EGTA buffer with Complete protease inhibitors (Roche). The brain was homogenized using a polytron followed by a dounce homogenizer and then centrifuged at 2,500g for 10 min at 4 °C. The supernatant was then centrifuged at 100,000g in a Sorvall TFT 80.4 rotor for 55 min at 4 °C and the pellet resuspended and sonicated in Tris-EGTA plus protease inhibitors. Proteins were separated on 12% acrylamide SDS–PAGE gels, transferred onto nitrocellulose, probed overnight with a commercially available rabbit monoclonal Na+ channel β1 subunit antibody developed by Cell Signaling Technology for L.L.I. (D9T5B, 1:750 dilution), then probed with a goat anti-rabbit secondary antibodies conjugated to horseradish peroxidase (Thermo Scientific, 1:1,000 dilution). Blots were incubated with West Femto enhanced chemiluminescent substrate (Thermo Scientific) and imaged using a LI-COR Odyssey system (LI-COR Biotechnology). Full images of western blots are presented in Supplementary Figs 32–34.
Data analysis
Data are presented as median and interquartile ranges or mean±s.e.m. Linear and nonlinear regressions were calculated with Prism 6.0c software, and parameters are reported in Supplementary Table 3. Statistical analysis was performed using SigmaPlot 11.0 software. Data were initially tested for normality (Shapiro–Wilk) and equal variance for assignment to parametric or non-parametric analysis. Parametric test included Student’s t-test or analysis of variance followed by Bonferroni test for non-paired comparison between two or among multiple groups, respectively. For paired statistical analysis, paired t-test or one-way repeated measures analysis of variance followed by Bonferroni test was employed for two groups or multiple comparisons, respectively. When normality or equal variance were not met, non-parametric analysis was performed using Mann–Whitney Rank sum test or Kruskal–Wallis one-way analysis of variance on ranks followed by Dunn’s method, for non-paired comparison between two or among multiple groups, respectively. Wilcoxon signed rank test or Friedman repeated measures analysis of variance on ranks was employed for paired comparison between two or among multiple groups, respectively. For categorical data analysis, Fisher’s exact or χ2-tests was used. P<0.05 was considered significant.
Additional information
How to cite this article: Signore, S. et al. Late Na+ current and protracted electrical recovery are critical determinants of the aging myopathy. Nat. Commun. 6:8803 doi: 10.1038/ncomms9803 (2015).
References
Schwartz, J. & Zipes, D. in Braunwald's Heart Disease: A Textbook of Cardiovascular Medicine 9th ed eds Bonow R., Mann D., Zipes D., Libby P. 1727–1753Saunders (2011).
Reardon, M. & Malik, M. QT interval change with age in an overtly healthy older population. Clin. Cardiol. 19, 949–952 (1996).
Roden, D. M. & Viswanathan, P. C. Genetics of acquired long QT syndrome. J. Clin. Invest. 115, 2025–2032 (2005).
Maeder, M. T. & Kaye, D. M. Heart failure with normal left ventricular ejection fraction. J. Am. Coll. Cardiol. 53, 905–918 (2009).
Zile, M. R. et al. Heart failure with a normal ejection fraction: is measurement of diastolic function necessary to make the diagnosis of diastolic heart failure? Circulation 104, 779–782 (2001).
Borlaug, B. A. & Paulus, W. J. Heart failure with preserved ejection fraction: pathophysiology, diagnosis, and treatment. Eur. Heart J. 32, 670–679 (2011).
Bers, D. M. Cardiac excitation-contraction coupling. Nature 415, 198–205 (2002).
Sah, R., Ramirez, R. J., Kaprielian, R. & Backx, P. H. Alterations in action potential profile enhance excitation-contraction coupling in rat cardiac myocytes. J. Physiol. 533, 201–214 (2001).
Sah, R. et al. Regulation of cardiac excitation-contraction coupling by action potential repolarization: role of the transient outward potassium current (I(to)). J. Physiol. 546, 5–18 (2003).
Janczewski, A. M., Spurgeon, H. A. & Lakatta, E. G. Action potential prolongation in cardiac myocytes of old rats is an adaptation to sustain youthful intracellular Ca2+ regulation. J. Mol. Cell. Cardiol. 34, 641–648 (2002).
Rota, M. et al. The young mouse heart is composed of myocytes heterogeneous in age and function. Circ. Res. 101, 387–399 (2007).
Signore, S. et al. Inositol 1, 4, 5-trisphosphate receptors and human left ventricular myocytes. Circulation 128, 1286–1297 (2013).
Nahrendorf, M. et al. Multimodal functional cardiac MRI in creatine kinase-deficient mice reveals subtle abnormalities in myocardial perfusion and mechanics. Am. J. Physiol. Heart Circ. Physiol. 290, H2516–H2521 (2006).
D'Souza, A. et al. Exercise training reduces resting heart rate via downregulation of the funny channel HCN4. Nat. Commun. 5, 3775 (2014).
White, M. et al. Age-related changes in beta-adrenergic neuroeffector systems in the human heart. Circulation 90, 1225–1238 (1994).
Xiao, R. P., Spurgeon, H. A., O'Connor, F. & Lakatta, E. G. Age-associated changes in beta-adrenergic modulation on rat cardiac excitation-contraction coupling. J. Clin. Invest. 94, 2051–2059 (1994).
Ram, R., Mickelsen, D. M., Theodoropoulos, C. & Blaxall, B. C. New approaches in small animal echocardiography: imaging the sounds of silence. Am. J. Physiol. Heart Circ. Physiol. 301, H1765–H1780 (2011).
Hao, X. et al. TGF-beta1-mediated fibrosis and ion channel remodeling are key mechanisms in producing the sinus node dysfunction associated with SCN5A deficiency and aging. Circ. Arrhythm. Electrophysiol. 4, 397–406 (2011).
Walker, K. E., Lakatta, E. G. & Houser, S. R. Age associated changes in membrane currents in rat ventricular myocytes. Cardiovasc. Res. 27, 1968–1977 (1993).
Wang, L. & Duff, H. J. Developmental changes in transient outward current in mouse ventricle. Circ. Res. 81, 120–127 (1997).
Nerbonne, J. M. & Kass, R. S. Molecular physiology of cardiac repolarization. Physiol. Rev. 85, 1205–1253 (2005).
Brouillette, J., Clark, R. B., Giles, W. R. & Fiset, C. Functional properties of K+ currents in adult mouse ventricular myocytes. J. Physiol. 559, 777–798 (2004).
Kaprielian, R. et al. Relationship between K+ channel down-regulation and [Ca2+]i in rat ventricular myocytes following myocardial infarction. J. Physiol. 517, (Pt 1): 229–245 (1999).
Liu, J., Kim, K. H., London, B., Morales, M. J. & Backx, P. H. Dissection of the voltage-activated potassium outward currents in adult mouse ventricular myocytes: I(to,f), I(to,s), I(K,slow1), I(K,slow2), and I(ss). Basic Res. Cardiol. 106, 189–204 (2011).
Wagner, S. et al. Reactive oxygen species-activated Ca/calmodulin kinase IIdelta is required for late I(Na) augmentation leading to cellular Na and Ca overload. Circ. Res. 108, 555–565 (2011).
Sossalla, S. et al. Ranolazine improves diastolic dysfunction in isolated myocardium from failing human hearts--role of late sodium current and intracellular ion accumulation. J. Mol. Cell. Cardiol. 45, 32–43 (2008).
Moreno, J. D. & Clancy, C. E. Pathophysiology of the cardiac late Na current and its potential as a drug target. J. Mol. Cell. Cardiol. 52, 608–619 (2012).
Gao, Y. et al. Inhibition of late sodium current by mexiletine: a novel pharmotherapeutical approach in timothy syndrome. Circ. Arrhythm. Electrophysiol. 6, 614–622 (2013).
Sicouri, S., Antzelevitch, D., Heilmann, C. & Antzelevitch, C. Effects of sodium channel block with mexiletine to reverse action potential prolongation in in vitro models of the long term QT syndrome. J. Cardiovasc. Electrophysiol. 8, 1280–1290 (1997).
Bouchard, R. A., Clark, R. B. & Giles, W. R. Effects of action potential duration on excitation-contraction coupling in rat ventricular myocytes. Action potential voltage-clamp measurements. Circ. Res. 76, 790–801 (1995).
Trafford, A. W., Diaz, M. E., Sibbring, G. C. & Eisner, D. A. Modulation of CICR has no maintained effect on systolic Ca2+: simultaneous measurements of sarcoplasmic reticulum and sarcolemmal Ca2+ fluxes in rat ventricular myocytes. J. Physiol. 522, (Pt 2): 259–270 (2000).
Rota, M. et al. Nuclear targeting of Akt enhances ventricular function and myocyte contractility. Circ. Res. 97, 1332–1341 (2005).
Voigt, N. et al. Enhanced sarcoplasmic reticulum Ca2+ leak and increased Na+-Ca2+ exchanger function underlie delayed afterdepolarizations in patients with chronic atrial fibrillation. Circulation 125, 2059–2070 (2012).
Maltsev, V. A., Silverman, N., Sabbah, H. N. & Undrovinas, A. I. Chronic heart failure slows late sodium current in human and canine ventricular myocytes: implications for repolarization variability. Eur. J. Heart Fail. 9, 219–227 (2007).
Undrovinas, N. A., Maltsev, V. A., Belardinelli, L., Sabbah, H. N. & Undrovinas, A. Late sodium current contributes to diastolic cell Ca2+ accumulation in chronic heart failure. J. Physiol. Sci. 60, 245–257 (2010).
Coppini, R. et al. Late sodium current inhibition reverses electromechanical dysfunction in human hypertrophic cardiomyopathy. Circulation 127, 575–584 (2013).
Zaza, A. & Rocchetti, M. The late Na+ current--origin and pathophysiological relevance. Cardiovasc Drugs Ther. 27, 61–68 (2013).
Vassalle, M. & Lin, C. I. Calcium overload and cardiac function. J. Biomed. Sci. 11, 542–565 (2004).
Fischer, T. H. et al. Late INa increases diastolic SR-Ca2+-leak in atrial myocardium by activating PKA and CaMKII. Cardiovasc. Res. 107, 184–196 (2015).
Lopez-Santiago, L. F. et al. Sodium channel Scn1b null mice exhibit prolonged QT and RR intervals. J. Mol. Cell. Cardiol. 43, 636–647 (2007).
Maltsev, V. A. & Undrovinas, A. Late sodium current in failing heart: friend or foe? Prog. Biophys. Mol. Biol. 96, 421–451 (2008).
Mishra, S. et al. Post-transcriptional silencing of SCN1B and SCN2B genes modulates late sodium current in cardiac myocytes from normal dogs and dogs with chronic heart failure. Am. J. Physiol. Heart Circ. Physiol. 301, H1596–H1605 (2011).
Chen-Izu, Y. et al. Na+ channel function, regulation, structure, trafficking and sequestration. J. Physiol. 593, 1347–1360 (2015).
Meadows, L. S. & Isom, L. L. Sodium channels as macromolecular complexes: implications for inherited arrhythmia syndromes. Cardiovasc. Res. 67, 448–458 (2005).
Abriel, H. & Kass, R. S. Regulation of the voltage-gated cardiac sodium channel Nav1.5 by interacting proteins. Trends Cardiovasc. Med. 15, 35–40 (2005).
Wagner, S. et al. Ca2+/calmodulin-dependent protein kinase II regulates cardiac Na+ channels. J. Clin. Invest. 116, 3127–3138 (2006).
Lakatta, E. G. & Levy, D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part II: the aging heart in health: links to heart disease. Circulation 107, 346–354 (2003).
Wilcox, J. E., Rosenberg, J., Vallakati, A., Gheorghiade, M. & Shah, S. J. Usefulness of electrocardiographic QT interval to predict left ventricular diastolic dysfunction. Am. J. Cardiol. 108, 1760–1766 (2011).
Antzelevitch, C. Ionic, molecular, and cellular bases of QT-interval prolongation and torsade de pointes. Europace 9, (Suppl 4): iv4–15 (2007).
Ozgen, N. et al. Determinants of CREB degradation and KChIP2 gene transcription in cardiac memory. Heart Rhythm 7, 964–970 (2010).
Lin, X. et al. Scn1b deletion leads to increased tetrodotoxin-sensitive sodium current, altered intracellular calcium homeostasis and arrhythmias in murine hearts. J. Physiol. 593, 1389–1407 (2015).
Kranias, E. G. & Hajjar, R. J. Modulation of cardiac contractility by the phospholamban/SERCA2a regulatome. Circ. Res. 110, 1646–1660 (2012).
Hagendorff, A., Schumacher, B., Kirchhoff, S., Luderitz, B. & Willecke, K. Conduction disturbances and increased atrial vulnerability in Connexin40-deficient mice analysed by transesophageal stimulation. Circulation 99, 1508–1515 (1999).
Knollmann, B. C. et al. Familial hypertrophic cardiomyopathy-linked mutant troponin T causes stress-induced ventricular tachycardia and Ca2+-dependent action potential remodeling. Circ. Res. 92, 428–436 (2003).
Ferreira-Martins, J. et al. Spontaneous calcium oscillations regulate human cardiac progenitor cell growth. Circ. Res. 105, 764–774 (2009).
Torella, D. et al. Cardiac stem cell and myocyte aging, heart failure, and insulin-like growth factor-1 overexpression. Circ. Res. 94, 514–524 (2004).
Cingolani, O. H. & Kass, D. A. Pressure-volume relation analysis of mouse ventricular function. Am. J. Physiol. Heart Circ. Physiol. 301, H2198–H2206 (2011).
Pacher, P., Nagayama, T., Mukhopadhyay, P., Batkai, S. & Kass, D. A. Measurement of cardiac function using pressure-volume conductance catheter technique in mice and rats. Nat. Protoc. 3, 1422–1434 (2008).
Pfeffer, M. A. et al. Myocardial infarct size and ventricular function in rats. Circ. Res. 44, 503–512 (1979).
Gehrmann, J. et al. Phenotypic screening for heart rate variability in the mouse. Am. J. Physiol. Heart Circ. Physiol. 279, H733–H740 (2000).
Saba, S., London, B. & Ganz, L. Autonomic blockade unmasks maturational differences in rate-dependent atrioventricular nodal conduction and facilitation in the mouse. J. Cardiovasc. Electrophysiol. 14, 191–195 (2003).
Lovelock, J. D. et al. Ranolazine improves cardiac diastolic dysfunction through modulation of myofilament calcium sensitivity. Circ. Res. 110, 841–850 (2012).
De Luca, A. et al. New potent mexiletine and tocainide analogues evaluated in vivo and in vitro as antimyotonic agents on the myotonic ADR mouse. Neuromuscul Disord 14, 405–416 (2004).
Goichberg, P. et al. The ephrin A1-EphA2 system promotes cardiac stem cell migration after infarction. Circ. Res. 108, 1071–1083 (2011).
Plotnikov, S. V., Millard, A. C., Campagnola, P. J. & Mohler, W. A. Characterization of the myosin-based source for second-harmonic generation from muscle sarcomeres. Biophys. J. 90, 693–703 (2006).
Rota, M. & Vassalle, M. Patch-clamp analysis in canine cardiac Purkinje cells of a novel sodium component in the pacemaker range. J. Physiol. 548, 147–165 (2003).
Sossalla, S. et al. Diastolic dysfunction and arrhythmias caused by overexpression of CaMKIIdelta(C) can be reversed by inhibition of late Na(+) current. Basic Res. Cardiol. 106, 263–272 (2011).
Wu, L. et al. Late sodium current contributes to the reverse rate-dependent effect of IKr inhibition on ventricular repolarization. Circulation 123, 1713–1720 (2011).
Tang, M. et al. Enhanced basal contractility but reduced excitation-contraction coupling efficiency and beta-adrenergic reserve of hearts with increased Cav1.2 activity. Am. J. Physiol. Heart Circ. Physiol. 299, H519–H528 (2010).
Vaidyanathan, R. et al. The ionic bases of the action potential in isolated mouse cardiac Purkinje cell. Heart Rhythm 10, 80–87 (2013).
Acknowledgements
We thank Dr Hugues Abriel and members of his laboratory at the University of Bern for providing the rabbit polyclonal Nav1.5 antibody, Ms Cuiqi Li and Dr Nicola Alesi at the Brigham and Women’s Hospital for histological and technical assistance, respectively. This work was supported by the National Institute of Health grants R01 HL114346, P01 AG043353, P01 HL092868, R01 AG037495, R01 HL111183, R01 AG037490, R01 HL105532, R01 AG044455, R37 HL081737 and R01 NS076752.
Author information
Authors and Affiliations
Contributions
S.S., A.S., P.G., A.L., P.A. and M.R. planned and designed the research; S.S., A.S., G.B., A.C., M.M., Y.Z., R.K., F.P., H.O’M., M.S., N.T., E.Z., C.A., C.M., K.I., B.F.S., J.K., L.L.I. and M.R. performed the research and contributed with analytic tools; S.S., A.S., G.B., A.C., M.M., Y.Z., R.K., F.P., H.O’M., M.S., E.Z., C.A., C.M., K.I., B.F.S., J.K., L.L.I., M.N. and M.R. analysed the data; S.S., A.S., P.A. and M.R. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
P.A. is a member of Autologous LLP. The remaining authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Figures 1-34 and Supplementary Tables 1-3 (PDF 5703 kb)
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Signore, S., Sorrentino, A., Borghetti, G. et al. Late Na+ current and protracted electrical recovery are critical determinants of the aging myopathy. Nat Commun 6, 8803 (2015). https://doi.org/10.1038/ncomms9803
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/ncomms9803
This article is cited by
-
Age-related increases in cardiac excitability, refractoriness and impulse conduction favor arrhythmogenesis in male rats
Pflügers Archiv - European Journal of Physiology (2023)
-
The common characteristics and mutual effects of heart failure and atrial fibrillation: initiation, progression, and outcome of the two aging-related heart diseases
Heart Failure Reviews (2022)
-
Is heart failure with preserved ejection fraction a ‘dementia’ of the heart?
Heart Failure Reviews (2022)
-
Chemogenetic generation of hydrogen peroxide in the heart induces severe cardiac dysfunction
Nature Communications (2018)
-
Single-cell analysis of the fate of c-kit-positive bone marrow cells
npj Regenerative Medicine (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.