Abstract
Lindlar catalysts comprising of palladium/calcium carbonate modified with lead acetate and quinoline are widely employed industrially for the partial hydrogenation of alkynes. However, their use is restricted, particularly for food, cosmetic and drug manufacture, due to the extremely toxic nature of lead, and the risk of its leaching from catalyst surface. In addition, the catalysts also exhibit poor selectivities in a number of cases. Here we report that a non-surface modification of palladium gives rise to the formation of an ultra-selective nanocatalyst. Boron atoms are found to take residence in palladium interstitial lattice sites with good chemical and thermal stability. This is favoured due to a strong host–guest electronic interaction when supported palladium nanoparticles are treated with a borane tetrahydrofuran solution. The adsorptive properties of palladium are modified by the subsurface boron atoms and display ultra-selectivity in a number of challenging alkyne hydrogenation reactions, which outclass the performance of Lindlar catalysts.
Similar content being viewed by others
Introduction
Catalytic partial hydrogenation of alkynes to alkenes is a key transformation in industrial organic synthesis1,2. It is established that a solid catalyst containing palladium (Pd) in the presence of hydrogen is the most preferable choice over stoichiometric chemical reagents and homogeneous catalysts in terms of green chemistry and reusability of the catalysts. However, the surface of unmodified supported Pd metal is highly unselective, which will lead to over-hydrogenation to undesired alkanes. Traditional approaches used to tune the surface and hence the chemical reactivity of Pd include: incorporating additives, modifying support materials or alloying with other metal atoms (and also core-shell) to Pd nanoparticles. As a result, Lindlar catalysts were developed with Pb and quinoline additives to modify the Pd sites and hinder the formation of Pd hydride in the proximity of the catalyst surface3. Lindlar catalysts have been demonstrated to be versatile and effective and remain to be most frequently used despite their discovery over 60 years ago. However, handling toxic Pb(OAc)2 during catalyst preparation, leaching of surface doped Pb element in the presence of substrates or products with multi-functional groups (such as drug candidates or biomass molecules), poor selectivity and limited catalyst robustness begin to restrict them from wider and new applications. In addition, the hydrogenation of monosubstituted alkynes to corresponding alkenes for the synthesis of synthons of functional polymers and total syntheses of complex molecules over Lindlar catalysts are still difficult and challenging1,2,3,4.
On the other hand, much less attention is paid to modifying Pd metal using light element atoms (defined here as being elements up to the third row elements) by placing them into the subsurface interstitial lattice sites within the metal lattice structure. The changes in lattice parameter and charge transfer between the host–guest may also alter the band structure, hence catalytic properties of the supported Pd metal5,6,7,8,9,10. It is therefore interesting to develop new Pd nanocatalysts modified with interstitial atoms as a greener alternative to Lindlar catalysts for selective hydrogenation reactions.
There have been few previous reports on Pd systems with interstitial atoms. By treating hydrogen gas over clean Pd at room temperature, it is known that hydrogen atoms can rapidly move into the Pd interstices due to it possessing the smallest atomic size, fast diffusion and strong chemisorption to form a Pd-β-hydride phase. Teschner et al.5 have recently shown that interstitial C (intC) produced in situ from adsorbed carbonaceous species at elevated temperature can be used to drastically alter the gas phase hydrogenation of alkynes. Subsurface O in Pd at high temperatures has also been demonstrated to be the kinetically active surface for CO oxidation in the high temperature regime6,7 and the selective epoxidation of ethylene over silver catalysts is also thought to involve the subsurface oxygen atoms8. Apart from the β-hydride, it was not possible to experimentally isolate these interstitial occupied materials that were produced in situ, let alone of control and tune their content: The major technical difficulty to isolation being that these materials only exist under transient conditions in a small quantity at elevated temperatures and under an atmosphere of reactive gas. It is important to note that the infiltration of light atoms into the close packed metal lattice, though they may fit into the interstitial holes, is usually a highly activated process that requires extreme conditions that could cause sintering of high surface metal nanoparticles. To overcome the sintering problem we have synthesized a Pd-intC nanocatalyst from glucose pyrolysis where the formed carbonaceous layer can rapidly encapsulate individual Pd nanoparticles for the interstice filling during the heat treatment process9,10. However, this approach requires a complex procedure to remove the surface carbon for subsequent catalytic applications, which can also reduce the interstitial carbon content. The interstitial carbon atom in the Pd lattice is unstable, which limits its potential applications in catalysis. It is thus highly desirable to develop supported Pd catalysts modified with other interstitial atoms that are both formed under ambient conditions and are stable under mild catalytic testing conditions. The relative atomic size, the nature of chemical species, as well as the enthalpy (electronic stabilization) and entropy of surface adsorption versus lattice entrapment are all expected to be important factors.
We have been interested in the synthesis of interstitial boron (intB) to modify the metal electronic structure, and consequent catalytic activity of Pd nanocatalysts. It is intriguing to note that limited volumes of literature exist on studying the effects of subsurface boron, with research being predominately focused on the study of high temperature ordered PdxBy alloys11,12,13,14,15,16. Although several routes to prepare interstitial B modified Pd (Pd-intB) materials have been documented17,18,19,20, none of the above have achieved a method that incorporates the criteria of low temperature synthesis, high degree of doping with intB and also importantly use a low toxicity B source equivalent, which are essential in modern catalysis synthesis. There have been some computational simulations for intB on other metal lattices and related catalysis studies, but versatile synthesis method, detailed material characterization, stability evaluation and more importantly the direct structure–activity relationship with important catalytic applications are still lacking.
Here, we explore the scope of interstitial chemistry by using borane tetrahydrofuran (BH3.THF) as a B source for the synthesis of a Pd-intB nanocatalyst and compare this with Lindlar catalysts within a number of industrially relevant, challenging partial hydrogenation reactions. A highly doped Pd-intB phase (up to 20% of atomic B) can be synthesized at low temperature, in steep contrast compared with the traditional high temperature process for bulk material synthesis21. It is demonstrated that the new supported and stable Pd-intB/C nanocatalyst displays ultra-selectivity for alkyne partial hydrogenations to cis-alkenes in both the liquid and gas phase without inducing hydrogenolysis for unnecessary bond cleavage, isomerization and double bond shift that commonly occur on conventional Lindlar catalyst surfaces within delicate fine chemical or pharmaceutical applications. The dramatic enhancement in selectivity for the chemical transformations over Pd is attributed to the presence of intB.
Results
Synthesis and characterization and materials stability
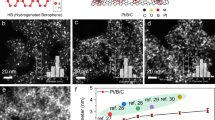
We began by exploring the addition of BH3.THF to a pre-formed sample of Pd/C (Fig. 1a, Supplementary Figs 3a,4,5, Supplementary Table 1, see Supplementary Notes and Supplementary Methods). A high concentration of BH3.THF (25 eq. mol B to mol Pd) was used to ensure maximum B incorporation. It was interesting to see that the B doped Pd samples were significantly different compared with their non-doped counterparts from the powder X-ray diffraction (PXRD) patterns (Supplementary Fig. 4). The difference was characterized by a definitive shift in XRD peaks to lower 2θ. Similarly, lattice expansion was also obtained by doping B to CaCO3 supported Pd, which showed that the nature of support did not seem to interfere the B incorporation (Supplementary Fig. 6). But the acidity and polarity of the support could affect hydrogenation in liquid phase so inert carbon support was mainly the focus in this work. Futher XRD characterziation using synchroton radiation at the Diamond Radiation Source, UK with the application of profile refinement clearly indicated the same unit cell lattice of face-centred cubic structure (Fm3m) but with an increase in lattice parameters. This effect is indicative of B interstitial occupation leading to a clearly observed expansion of the Pd metal lattice at room temperature. An unmodified Pd phase could no longer be observed when a further heat treatment at 200 °C in N2 was used (Supplementary Table 2). Additionally, EDX line scans of PdB particles under our analytical transmission electron microscope (Fig. 1b) clearly show the higher content B signal associated with Pd signal of selected particles from the background. This suggests that B is taken up by the Pd nanoparticle. 11B ssNMR also indicated the presence of both boron species on surface as well as in the interstitial sites of Pd after the BH3.THF treatment (Supplementary Fig. 7). Solid solutions of boron atoms in the interstitial sites of Pd fcc lattice with a range of composition and structure prepared by the extremely high temperature preparation method that utilizes excess arc melted B atoms to infiltrate into Pd foils have been extensively studied17. We have estimated the occupancy of interstitial sites from the lattice expansion to be ca. 20% (Supplementary Equation 1 and Supplementary Table 1), which is comparable to the saturated value produced from the reported high temperature method17. It should also be noted that the intrinsic Pd fcc phase was retained with no ordered alloys observed at room temperature. We attribute the efficient low temperature process for the production of interstitial B atoms from BH3.THF in contact with the supported Pd nanoparticle surface partly to an entropic contribution. The adsorbed B atoms are formed initially on the Pd surface with the subsequent evolution of H2 gas from the precursor. A strong driving force to move the surface B atoms deep into the Pd lattice is therefore expected in this case. In contrast to this low temperature process, only ~15% site occupancy of intC was achieved by our Pd nanoparticles encapsulated by glucose derived carbonaceous coating at high temperature regardless of the particle size, where the carbon saturation was reached13. It is interesting to note that comparison of the atomic radii between the light elements (H, B, C, N and O), B is the largest atom and yet the formation of Pd-intB with atomic fraction is remarkably higher than C and causing larger lattice expansion (~3.6% versus ~2.3% in lattice parameter) and can be carried out using such a mild temperature treatment.
(a) Lattice parameter, a0, measured using the average of all observable fcc Pd peaks from XRD (derived from lab-based X-ray diffractometer) normalized against the Al peaks from the sample cell. The error bar in lattice parameter at each temperature represents experimental error based on 3–5 repeated measurements. (b) A detailed EDX line scan of two Pd containing particles from fresh Pd(B)/C sample and corresponding X-rays counts of Pd(K) and B(K). It can be clearly seen that the B signals mirror to those of Pd showing that the signals are arisen from the Pd(B) particles (the B signal is higher than background suggesting that the support takes up B species, (refer to XPS in Supplementary Figs 1,2)). Scale bar, 50 nm.
In the late 1930s, Hägg and coworkers predicted the maximum size of interstitial atoms that could be accommodated in a lattice, with the assumptions that the interstitial atoms are totally inert with no interaction with host atoms and do not disrupt lattice packing as a truly solid solution. Theoretically, no solid solution should ever be formed between Pd and B, according to the Hägg’s criterion, the critical atomic radius ratio between the light element (ri) and the metal (rm) is satisfied only if the atomic radius ratio (ri/rm) is <0.59 (rB/rPd=0.67)17.
Apparently, the hard sphere model is inconsistent with the observed volume–interstitial content relationship for the interstitial solid solution Pd-intB: a significant, negative deviation from the hard sphere model prediction (volume contraction) occurs. It was concluded that the chemical (electronic) interaction appears to govern the interstitial solid solution formation rather than the elastic interaction as characterized by the Hägg atomic radius ratio or the volume size factor. Furthermore, it is energetically disfavoured to expand the metal lattice and therefore apart from the entropic contribution, extra energy stabilization must be needed for the migration of such light atoms from metal surface to interstitial sites (further discussion on Hagg’s and King’s size criteria is in Supplementary Discussion). It is believed that this extra stabilization must come from a direct electronic interaction with the host lattice atoms, the mixing of the B s-p orbitals with Pd d-orbitals (DFT calculations shown later).
Consequently, we investigated the stability of the new Pd-intB phase by determining the amounts of intB retained in Pd after exposure to a series of reactive gases, as measured by their shifts in lattice parameter (Fig. 1a and Supplementary Figs 4,5,8–10 and calculation method used). Interestingly, significant differences were observed when the Pd-intB/C was heat treated under different gaseous atmospheres. It can be seen that no metallic Pd peak (with an unmodified lattice parameter) was evident under N2 at 200 °C, where maximum boron incorporation (lattice expansion of 3.6%) was maintained. When Pd-intB/C was treated in air, a clear loss of boron was observed at their analogous temperatures. The content of IntB was attenuated under oxidising conditions but there was only marginal loss of boron in H2 atmosphere at above 200 °C (see temperature programmed reduction with mass spectrometry (Supplementary Fig. 11). Nevertheless, there was no loss in B under a flowing stream of hydrogen over the temperature range of 100–150 °C at prolonged time of testing, which suggests Pd-intB/C catalyst is stable to be used under the conditions for most selective chemical transformations of fine and speciality chemicals, as we targeted it for. On comparing these values with the class of Pd-intC catalysts studied22,23, it is apparent that the Pd-intB nanocatalysts exhibit much higher thermal stability under a reducing atmosphere than the Pd-intC catalyst, as all the intC are readily converted to CH4 at 150 °C under a reducing atmosphere. Therefore, the higher apparent thermal stability of Pd-intB and higher degree of interstitial filling at milder conditions could be ascribed to a corresponding higher degree of orbital interaction, leading to its increased stability compared with Pd-intC. It is apparent that Pd-intB/C can be handled in air at or below 100 °C without phase segregation; however, at more elevated temperatures significant decomposition of the Pd-intB phase was observed. Heat treating the catalyst in air at ≥200 °C resulted in ejection of intB with simultaneously reformation of metallic Pd, boron migration from subsurface is likely to have been induced through oxidation of boron atoms with adsorbed oxygen that remained on the surface of Pd (see temperature programmed oxidation with mass spectrometry, Supplementary Figs 12,13). Chemisorbed oxygen has a propensity to induce phase segregation, as there is a stronger binding affinity of B for O than Pd24. As a result, one should be cautious of examining this type of catalysts for reactions under different gas atmospheres at unsustainable high temperatures.
Extended X-ray absorption fine structure (EXAFS) fitted parameters are shown in Supplementary Equation 1,3 and Supplementary Table 3. It can be seen from the fitted data that addition of B clearly leads to an expansion of the average Pd–Pd distance, as evidenced by the reduced amplitudes from the EXAFS oscillations (Supplementary Fig. 14). The degree of lattice expansion suggests that B is indeed occupied in the octahedral holes of Pd, this fact is consistent to the PXRD data25. The structure of Pd remained in the fcc structure in agreement with the PXRD. The interatomic distance increased upon addition of B in Pd. This result was in line with the previous study where Pd foil was homogenized with atomic B followed by rapid quenching to form Pd-B25. The coordination number apparently decreased on addition of B, albeit the lower value for Pd/C falls within the upper value for Pd-intB/C. The amount of B in the sample determined from the lattice parameter was estimated at 11.3% in contrast with 20% B estimated by PXRD, which could reflect the difference in sensitivity to Pd sizes and their variations in B loadings by the two measurements. It is noted that the fitting of EXAFS data could be marginally improved by adding an extra-shell of B (Pd–B interaction) but this analysis was not included with taking experimental errors into consideration due to B being an extremely poor X-ray absorption (despite the high B/Pd signals shown in the EDX line scans of the Pd(B) particles (Supplementary Fig. 15).
Catalytic performance
It is important to establish whether these new Pd-intB nanoparticles supported on activated carbon or calcium carbonate with electronically-modified Pd by the subsurface B atoms can be of any significance for selective hydrogenation transformations under mild conditions. Selective partial hydrogenation of fine and speciality chemicals in the liquid phase was chosen as these present significant challenges for ultra-selectivity (delicate molecules may easily undergo side reactions that need to be eliminated).
First, partial hydrogenation of chloro-nitrobenzene (CNB) to chloroaniline (CAN) without hydrodechlorination (to avoid hydrogenolysis of the facile aryl-Cl bond is a key challenge) was investigated. This hydrogenation represents an important class of chemical transformation towards the manufacture of dyes, pharmaceuticals, polymers, herbicides and pesticides26. Most studies in the literature have focused on using Pt-based catalysts to achieve high CNB hydrogenation while at the same time maintaining low rates of hydrodechlorination27,28,29. Meanwhile, conventional Pd based catalysts generally offer significantly lower selectivity due to aryl-chloride bond cleavage30,31,32. Furthermore, the poor activity and severe leaching of Pb to form PbCl2 render Lindlar catalysts totally unsuitable for this application1,32. However, there is a strong economic advantage to use Pd instead of Pt in this application, due to the lower costs of the former. Recently, studies have been devoted on maximizing CAN selectivity either by, to name but a few, alloying7,33, incorporating additives34 or using different metal supports to Pd26. Therefore, the versatility of this new class of catalyst was investigated for the partial hydrogenation of o-CNB to o-CAN (Supplementary Fig. 16) as compared with a commercial 5% Pd/C, a non-modified 3% Pd/C and a corresponding 3% Pd-intB/C catalyst. Figure 2a shows the H2 uptake curves for those catalysts tested under industrially applicable conditions. A higher initial activity was observed for the commercial 5% Pd/C and is attributed to the smaller particle sizes (~5 nm) compared with the non-modified 3% Pd/C catalysts. The initial activity for 3% Pd-intB/C was lower than that of the unmodified catalyst, indicating a partial loss of surface area after B addition (Supplementary Table 4). However, the large difference in the initial activity between Pd/C and Pd-intB/C could not be entirely due to the loss in metal sites but the modified surface electronic structure by B incorporation. Figure 2b and Supplementary Table 5 shows the gas chromatography analysis at the rate change in H2 uptake and at the end of the reaction. Notice that the commercial 5% Pd/C sample gave only 42.8% selectivity towards o-CAN. The catalyst appeared to yield excessive hydrodechlorination as evidenced by the higher selectivities towards aniline (31.7%) and unknown products (1.8% unkown) being isolated as the main by-products. Analysis at the end of the reaction led to almost no selectivity towards the partial hydrogenated product o-CAN (1.8%), which was accompanied by observation of higher boiling point diazo products due to excessive adsorption of intermediate(s) and catalysis on surface. In contrast, a higher selectivity to o-CAN (81.3%) was obtained over 3% Pd/C with a larger average Pd particle size (13 nm). Upon comparing the 3% Pd-intB/C with non-modified 3% Pd/C of a similar particle size but with the B inclusion (Supplementary Table 5), it was clear that Pd-intB gave a much enhanced selectivity to o-CAN of 95.4%, the difference in their selectivity became larger towards the end of the reaction (an increase to 27%). In addition, no formation of higher boiling by-products was observed for the Pd-intB/C. This result clearly demonstrates that the Pd-intB gives the ability to suppress the delicate hydrodechlorination pathway despite the failure of using Lindlar catalysts, which may be used as an alternative for Pt based catalysts in the chemoselective hydrogenation of o-CNB to o-CAN.
(a) H2 uptake curves for hydrogenation of o-CNB on: (i) commercial 5% Pd/C (red); (ii) non-modified 3% Pd/C (black); (iii) 3% Pd-intB/C (blue). Conditions: 2.46 mmol octane and o-CNB, 3.80 μmol catalysts, octane as internal standard, EtOH, 3 bar H2, 50 °C, 1,200 r.p.m., HEL ChemSCAN II Reactor. (b) GC results in the hydrogenation of o-CNB. Stop: sample collected before rate change in the H2 uptake (at 190, 170 and 155 ml H2 uptake for commercial 5% Pd/C, non-modified 3% Pd/C and 3% Pd-intB/C, respectively). End: sample collected at the end of H2 uptake. All samples were at 100% conversion of o-CNB; H2 uptake value of ~65 ml for 1 mol equiv. o-CNB.
The stereoselective partial hydrogenation of 3-hexyn-1-ol to the corresponding cis-hexen-1-ol (CHEL) was also chosen for the study as this typical reaction has significant importance in the fragrance industry towards the manufacturing of leaf fragrance alcohol (Supplementary Fig. 17 and Supplementary Table 6)35. Even trace levels of undesirable over-hydrogenated products and other isomers would greatly alter the aroma of the mixture. The reaction progress was monitored by measuring the H2 uptake against time under the same conditions (Fig. 3a). It was found that all catalysts gave good activity, reaching the semi-hydrogenation point after 20 min. An induction period was observed in the initial stages of H2 uptake for the Lindlar catalyst, as characterized by the curve having a slight parabolic shape that suggests that the catalyst was initially in the oxidized state36. Aliquots were taken at points where the reaction reached the semi-hydrogenation theoretical uptake point (1 mol equiv. of H2 uptake with respect to alkyne) and also at the end of the reaction, for analysis by gas chromatography (Fig. 3b). When comparing the catalysts, it was found that the 3% Pd-intB/C was again the most selective towards CHEL (98%) as characterized by the very high cis/trans ratio at the half H2 uptake point (53.5). It is interesting that virtually no over-hydrogenation products were observed for Pd-intB/C as no hexanol (HAL) was detected. The selectivity towards CHEL and cis/trans ratio are apparently higher than that of Pd-intC catalysts we reported earlier (91.9% CHEL, cis/trans ratio of 10.8) under the comparable conditions9,10 suggesting that intB is more effective than intC in subsurface modification of Pd for this reaction. On the other hand, both 3% Pd/C and surface poisoned Lindlar catalyst suffered from the significant over-hydrogenation to HAL as well as a rapid isomerization to trans-hexen-1-ol. The adsorption strength of alkyne on metal surface is generally much higher than corresponding alkene molecules, thus modification by interstitial atoms or surface dopings to reduce the adsorptive ability of Pd are known to be beneficial to give higher selectivity towards alkenes (cis and trans) at high conversion (the metal surface is primarily covered with alkyne molecules). But after the conversion of alkyne, the fine tuning of metal surface is very important to reduce the further conversion of desirable cis alkene by weakening its absorption (reduction in isomerization reactions to trans or double bond shift of the adsorbed molecule). It is clear from our results that the use of the longer contact time in hydrogen in the batch reactor after the half hydrogen uptake allowed for further isomerizations to take place for all the catalysts but the high cis/trans ratio at the end of experiment in the case of Pd-intB/C (cis/trans ratio=25.4) clearly suggests that the extent of isomerization over this catalyst is not prominent. Comparatively, at the end of reaction, the Lindlar catalyst gave 43.9% CHEL with 30.6% towards HAL with cis/trans ratio of 1.4. Thus, the Pd-intB/C gave much reduced isomerization rate compared with the non-modified 3% Pd/C with the Lindlar catalyst, the highest of all three catalysts. An increase in the rates of over-hydrogenation and isomerization for Pd/C and Lindlar catalyst is attributed to the uncompromized stronger adsorption of the partial hydrogenated product on the catalyst surfaces due to characteristic d-band positions (see DFT calculations) and partly to the hydrophilic nature of support with higher affinity for hydrophilic substrate. Whereas the Pd-intB/C (non-hydrophilic) with modified electronic band structure by the boron atoms greatly reduced the residence time for substrate adsorption (weaker adsorption) and hence importantly much lower isomerization rates.
(a) Hydrogen uptake curves in the hydrogenation of 3-hexyn-1-ol for: (i) non-modified 3% Pd/C (black); (ii) Pd-intB/C (blue); (iii) Lindlar’s 5% PdPb/CaCO3 (red). Conditions: 30 mmol 1,4-dioxane and 3-hexyn-1-ol, 2.80 μmol catalyst in EtOH, 3 bar H2, 30 °C, 400 r.p.m., Parr autoclave. H2 uptake value of ~650 ml for 1 mol equiv. to alkyne. (b) GC results for the stereoselective hydrogenation of 3-hexyn-1-ol. Stop: sample collected before the change in hydrogen uptake. End: sample collected at the end of hydrogen uptake. THEL, trans-hexen1-ol; CHEL, cis-hexen-1-ol; HAL, 1-hexanol. Yellow dot represents percent of conversion.
Hydrogenation of non-polar substituted alkyne (for example, diphenylacetylene (DPA)) was also examined in ethanol over both Lindlar catalyst and 3% Pd-intB/C (Fig. 4). The reaction progress was again followed by monitoring the H2 uptake (Fig. 4, conditions: HEL ChemSCAN, three bar H2 (excess H2) 30 °C, 2.50 μmol catalyst, 0.5 M alkyne in 5 ml EtOH, 1,200 r.p.m., 1,000:1 substrate to metal ratio). The rate difference between H2 uptakes before/after 1 mol equiv. of H2 consumption (~62 ml of H2 in this case) gave an indication of semi-hydrogenation selectivity to the alkene, as aforementioned (only the semi- and fully-hydrogenated products were detected). The Lindlar catalyst exhibited almost the same initial activities and selectivities for this alkyne hydrogenation as 3% Pd-intB/C at the inflection point of 65 ml where 1 mol equiv. H2 consumption was reached (89.7% DPA conversion, 94.3% selectivity to stilbene (alkene products with cis/trans=32.7) and 5.7% selectivity to diphenylethane (alkane) for Lindlar catalyst). However, a much poorer hydrogenation selectivity was observed towards stilbene at longer time as the catalyst continued to hydrogenate towards the saturated alkane beyond the semi-hydrogenation point until reaching the total consumption of 2 mol equiv. of H2 (~124 ml of H2) with virtually 100% yield to diphenylethane. The over-hydrogenation on corresponding terminal alkyne, the phenylacetylene (PA) was even more prominent as there was no inflection near the equivalent semi-hydrogenation region to quench the reaction to alkene (styrene) in the hydrogenation curve (Fig. 4) but the hydrogenation rapidly proceeded to completion, giving 100% yield to alkane product (ethyl-benzene). This is not surprising as non-polar mono-substituted alkynes, as previously stated, are in general significantly more difficult to hydrogenate to unsaturated alkenes due to their smaller difference in adsorption strength on the metal surface4. It is noted that unsatisfactory results were obtained with the use of Lindlar catalyst over such non-polar alkynes hydrogenation in alcoholic solvents1. An elaborate set-up to restrict access of hydrogen gas and to monitor the reaction progress is commonly required to reduce the excessive over-reduction to alkanes. In addition, delicate adjustment of surface concentration of lead acetate, depending on nature of substrate, to avoid over poisoning the Pd/CaCO3 catalyst is needed. It is often observed that hydrogenations over Lindlar catalysts proceeded not at all or only very slowly1.
(a) Hydrogen uptake curves in the hydrogenation of (i) Diphenylacetylene (DPA) over Pd-intB/C catalyst (black); (ii) DPA over Lindlar’s catalyst (red); (iii) Phenylacetylene (PA) over Pd-intB/C catalyst (green); (iv) PA over Lindlar’s catalyst (blue). Conditions: 2.50 mmol alkyne, 2.50 μmol catalyst, EtOH, 3 bar H2, 30 °C, 1,200 r.p.m. H2 uptake value of ~62 ml for 1 mole equiv. to alkyne. (b) Structure of substrate molecule (top: DPA, bottom: PA).
On the other hand, for 3% Pd-intB/C catalyst with intrinsic interstitial B modification, at the equivalent 1 mol of hydrogen uptake there was slightly higher degree of conversion of DPA to stilbene with higher cis/trans ratio (91.4% DPA conversion, 93.8% selectivity to stilbene (cis/trans=35.1) and 6.1% selectivity to diphenylethane). Clearly in accordance with Fig. 4, the rate of over-hydrogenation of the DPA molecule beyond 1 mol hydrogen equivalent was very slow, giving similar stilbene selectivity even in excess of hydrogen atmosphere at prolonged time. In addition, the over-hydrogenation of the more challenge terminal PA alkene over the 3% Pd-intB/C catalyst was still continued in excess hydrogen (at 1 mol H2 equivalent, 100% PA conversion, 92.9% selectivity to styrene, 7.1% selectivity to ethyl-benzene until reaching 2 mol H2 equivalent with 100% yield to ethyl-benzene) but at a much lower rate as seen from the slower H2 uptake curve, which can be alleviated by limiting the access of hydrogen or/and time due to a clear difference in the rates for the first and second hydrogenations. By using CaCO3 as a support, though it is more polar than that of carbon support, a similar trend of 5% Pd-intB/CaCO3 was also obtained compared with 5% Pd/CaCO3 for the slower rate for over-hydrogenation of alkene in the hydrogenation of DPA or PA (Supplementary Fig. 18 and Supplementary Table 7). Again, the hydrogenation of DPA molecule beyond 1 mol hydrogen equivalent was very slow, giving higher stilbene selectivity and cis/trans ratio than that of the boron absent Pd sample even in excess of hydrogen atmosphere at prolonged time.
These results clearly demonstrate that the boron modified catalysts despite the use of different supports indeed show a dramatic, beneficial effect of ultra-selectivity in typical partial hydrogenation reactions, even for highly challenging non-polar substrates in liquid phase. This type of catalyst did not seem to deactivate in given short testing time (within few hours) in lab scale and there was also no significant loss in B content in post-mortal catalyst analysis (Supplementary Fig. 15).
Surface and subsurface contributions
The overall boron modification effect leading to ultraselectivity towards partial hydrogenated products may be derived from a number of contributing factors. These may include surface electronic effects, interstitial electronic effects and selective site blockage by the boron modification. Krawczyk et al.37 studied the effect of surface B (borides and borates) in the hydrogenation of alkynes. They observed a degree of promoted alkene selectivity over alkane products by boron adspecies, which suggests that a selective surface blockage mechanism can be taken place. As a result, we have briefly studied the hydrogenation of 4-octyne (Supplementary Equations 12–18 and Supplementary Fig. 19,20) over Pd-intB/C with and without interstitial boron (removal of intB selectively by 200 °C treatment in O2 as indicated by PXRD but XPS indicated that the catalyst contained surface boron species). Remarkably, the rate ratios for the catalyst with intB removed led to a substantial decreased in selectivity (Supplementary Table 8). This clearly suggests that intB has a significant role on minimizing over-hydrogenation and isomerization processes.
DFT calculations
As previously shown, placing boron atoms in the interstices of Pd lattice can create simultaneous electronic modification to the Pd band structure by lattice expansion and electronic interaction between intimate atoms. To appreciate their contributions we have turned to DFT calculations. Figure 5a illustrates the density of states for our clean surfaces, the top part of the panel shows the projected d-band of the surface Pd in clean Pd(111), whereas the bottom part of the panel shows the d-band of Pd in the absence of the p-block element (but frozen with the displacement induced by the p-block element), the d-band of the surface Pd in Pd/B and finally the p- and s-band of boron. This allows one to consider, firstly, the influence of expansion in the Pd lattice on the d-band, where as expected the d-band shifts up and narrows (d-band centre/width (eV). −1.37/1.00 as compared with −1.52/1.14 (pure)). This band uplift should in principle strengthen the adsorption strength of the substrate/intermediate molecules on surface38, which apparently contradicts to what was observed experimentally (much weakened in adsorption). On the other hand, the influence of introducing interstitial boron appeared to facilitate an effective overlap of d- and p-bands leading to downshifts the position of d-band centre and a corresponding band broadening (d-band centre/width (eV). −2.05/1.56 (intB)). One would expect a similar electronic effect between boron and Pd on surface but the extensive electronic interaction of interstitial boron with parent Pd lattice is thought to be more substantial. Figure 5b clearly shows the electron enrichment of subsurface region of intB atoms indicating that it is energetically favourable to place B atom in octahedron site as a strong host–guest electronic interaction. Thus, it is believed that the overriding effect is the Pd d-band broadening and down-shifting with a good degree of state mixing from the interstitial boron atoms in sub-layers, which provide a strong stabilization to this solid solution system. The occupancy of the interstitial boron atoms can displace the H atoms (Pd hydride) at the same sites to reduce undesirable over-hydrogenation by subsurface hydrogen39 and dramatically attenuate the adsorptivity of the metal surface to achieve ultra-selectivity in partial hydrogenation. A similar effect for electronic modification of Pd by surface Pb in Lindlar catalysts to destabilize the subsurface hydride formation has been proposed3. But, the conversion of 3-hexyn-1-ol to CHEL with higher selectivity over Pd-intB/C than Lindlar catalyst (Fig. 3) clearly suggests the more effectiveness of subsurface B modification.
(a) Density of states diagram of pure Pd (upper part of panel); d-states of Pd with frozen displacement (cyan); d-states of Pd with intB (blue); p-states of B in Pd (red); s-states of B in Pd (green; lower part of panel); (b) an electron density difference isosurface plot showing local electron richer (light blue) and poorer regions (yellow) of Pd atoms (blue) with intB atoms (pink) due to states-mixing (−'ve/+'ve density units=±0.05 electron per angstrom cube); (c) potential energy profile of 3-hexyn-1-ol adsorbed on a (2x4)-Pd(111) unit cells of 3-atomic layers on clean Pd and Pd-intB and (d) isosurface of isolated molecular Highest Occupied Molecular Orbital/Lowest Unoccupied Molecular Orbital (HOMO/LUMO) (blue/yellow, respectively) superimposed on the adsorbate/surface structure (units=0.3 electron per angstrom cube).
Discussion
We have also calculated simplified reaction profiles for the partial hydrogenation of 3-hexyn-1-ol to the corresponding cis and to trans forms in order to appreciate the high cis/trans ratio over the Pd-intB (Fig. 5c). In the gas phase, the total energy calculations yielded an energetic preference from the substrate hydrogenation to trans conformation by 0.14 eV than the cis product, within the realms of DFT error (±0.1 eV) that corresponds to an equilibrium constant of 225 (300 K) between trans and cis. However, if we take the substrate molecule to the surface, it will then favourably be converted to cis conformation in the energetic sequence of Eads(Pd)>Eads(Pd-intB). As the adsorptive strength of Pd-intB surface is much weaker than Pd/C (broadening and downshift of d-band, see Fig. 5b,d), which will encourage desorption of the cis-alkene to take place (following similar arguments by Norskov and co-workers38 for selective acetylene hydrogenation40). Notice that there is a breaking degeneracy to the favouring of a cis species by 0.17 eV corresponding to an equilibrium constant of 706 (300 K), which is a sharp contrast with the noncatalysed gas phase that favours the trans form (Supplementary Equations 4–11). Given the stability and similarly in calculated cis and trans energy and the existence of lower H barriers on pure Pd system (via β-hydride) as well as Lindlar catalyst (much smaller energetic preference than Pd-intB is anticipated), we would expect to see their higher degree of isomerization as observed experimentally (see Fig. 3).
In conclusion, we demonstrate that a low temperature incorporation of boron atoms into the metal lattice of supported Pd nanoparticles is favourable due to the strong electronic interaction within the host–guest sites using BH3.THF as a modifying reagent. This simple chemical modification can be applied directly to existing metal supported catalysts. It is also shown for the first time that the Pd metal modified with subsurface boron atoms as a catalyst can give ultra-selectivity in selected partial hydrogenation reactions, outperforming those of Lindlar catalysts and Pd-intC in the liquid phase owing to the stronger electronic perturbation to the parent metal atoms. With regards to the growing demands for greener catalysts in partial hydrogenations and the limitations of Lindlar catalysts, the subsurface modification of Pd with boron atoms may thus find new uses in catalytic applications for fine-tuning adsorptivity/reactivity over the conventional surface methods.
Methods
Synthesis of Pd/C
A known amount of palladium (II) nitrate solution (15.11% Pd assay, Johnson Matthey) was dissolved in n-butanol and impregnated dropwise into a butanoic solution of Norit GSX activated carbon (Johnson Matthey) to achieve the desired theoretical metal loading. n-butanol was used as solvent because the hydrophobic properties of water may have a detrimental effect to the final metal particle size. This was stirred overnight after which the suspension was filtered and washed with copious amount of water followed by ethanol. A non orange coloured filtrate indicated anchoring of palladium onto the activated carbon support. The catalyst was dried at 80 °C in the oven overnight. Reduction of the dried catalyst was conducted in a 50:50 hydrogen to nitrogen atmosphere at a flow rate of 60 ml min−1. The furnace was programmed using a ramp rate of 5 °C min−1 up to 500 °C and held for 1 h. The furnace was then cooled back to room temperature. Supplementary Fig. 3 shows the transmission electron microscope image of Pd/C after reduction. The average metal crystallite size was found to be 13±3 nm.
Synthesis of Pd-intB/C
The preparation of Pd-intB/C nanocatalysts was performed in a three-neck round bottom flask equipped with hydrogen and nitrogen switch, rubber bung and an oil bubbler. Typically, 3% Pd/C (250 mg, 0.07 mmol) was added, purged with nitrogen gas and then evacuated overnight for 20 h in vacuum at 80 °C to ensure that any residual oxygen or moisture do not prematurely oxidize the BH3.THF added at later time. Catalyst was then pretreated in situ at 100 °C for 1 h then cooled down to room temperature in hydrogen. 2 ml of tetrahydrofuran was added to pre-wet the catalyst followed by a dose of BH3.THF solution (1.8 ml, 1.76 mmol, Aldrich). The mole ratio of Pd to BH3.THF was 1:25. The flask was warmed to 40 °C to aid solvent evaporation before measurement.
Additional information
How to cite this article: Chan, C. W. A. et al. Interstitial modification of palladium nanoparticles with boron atoms as a green catalyst for selective hydrogenation. Nat. Commun. 5:5787 doi: 10.1038/ncomms6787 (2014).
Change history
23 February 2015
A correction has been published and is appended to both the HTML and PDF versions of this paper. The error has not been fixed in the paper.
References
Nishimura, S. Handbook of Heterogeneous Catalytic Hydrogenation for Organic Synthesis Wiley (2001).
Crespo-Quesada, M., Yarulin, A., Jin, M., Xia, Y. & Kiwi-Minsker, L. Structure sensitivity of alkynol hydrogenation on shape- and size-controlled palladium nanocrystals: which sites are most active and selective? J. Am. Chem. Soc. 133, 12787–12794 (2011).
García-Mota, M. et al. A density functional theory study of the ‘mythic’ Lindlar hydrogenation catalyst. Theor. Chem. Acc. 128, 663–673 (2011).
Yabe, Y. et al. Development of a palladium on boron nitride catalyst and its application to the semihydrogenation of alkynes. Adv. Synth. Catal. 354, 1264–1268 (2012).
Teschner, D. et al. The roles of subsurface carbon and hydrogen in palladium-catalyzed alkyne hydrogenation. Science 320, 86–89 (2008).
Nagarajan, S., Thirunavukkarasu, K. & Gopinath, C. S. A revisit to carbon monoxide oxidation on Pd(111) surfaces. J. Phys. Chem. C 113, 7385–7397 (2009).
Gopinath, C. S., Thirunavukkarasu, K. & Nagarajan, S. Kinetic evidence for the influence of subsurface oxygen on palladium surfaces towards CO oxidation at high temperatures. Chem. Asian J. 4, 74–80 (2009).
Van den Hoek, P. J., Baerends, E. J. & Van Santen, R. A. Ethylene epoxidation on silver(110): the role of subsurface oxygen. J. Phys. Chem. 93, 6469–6475 (1989).
Chan, C. W. A., Tam, K. Y., Cookson, J., Bishop, P. & Tsang, S. C. Palladium with interstitial carbon atoms as a catalyst for ultraselective hydrogenation in the liquid phase. Catal. Sci. Technol. 1, 1584–1592 (2011).
Chan, C. W. A. et al. New environmentally friendly catalysts containing Pd-interstitial carbon made from Pd-glucose precursors for ultraselective hydrogenations in the liquid phase. Chem. Commun. 47, 7971–7973 (2011).
Alqasmi, R. A., Brodowsky, H. & Schaller, H.-J. Constitution of palladium-boron alloys. Zur Konstitution von Palladium-Bor-Legierungen 73, 331–334 (1982).
Beck, M., Ellner, M. & Mittemeijer, E. J. Powder diffraction data for borides Pd3B and Pd5B2 and the formation of an amorphous boride Pd2B. Powder Diff. 16, 98–101 (2001).
Beck, M., Ellner, M. & Mittemeijer, E. J. The structure of the palladium-rich boride Pd5B (Pd16B3). Zeitschrift Fur Kristallographie 216, 591–594 (2001).
Alqasmi, R. A., Brodowsky, H. & Schaller, H. J. On the thermodynamics of Pd-B alloys. Zeitschrift Fur Metallkunde 77, 637–642 (1986).
Husemann, H. & Brodowsk, H. Magnetic susceptibility and hydrogen absorption of palladium-boron alloys. Zeitschrift Fur Naturforschung Part a-Astrophysik Physik Und Physikalische Chemie A 23, 1693 -& (1968).
Brodowsky, H. & Schaller, H. J. Miscibility gaps in Pd-B-alloys. Berichte Der Bunsen-Gesellschaft-Physical Chemistry Chemical Physics 80, 656–661 (1976).
Beck, M., Ellner, M. & Mittemeijer, E. J. The formation of interstitial solid solutions based on solvents showing the fcc structure: elastic versus chemical interaction. Acta. Mater. 49, 985–993 (2001).
Liao, P., Spear, K. & Schlesinger, M. The B-Pd (boron-palladium) system. J. Phase Equilib. 17, 340–345 (1996).
Krawczyk, M. Decomposition of diborane on Pd(111): thermal and chemical behaviour of surface boron. Appl. Surf. Sci. 135, 209–217 (1998).
Wang, J.-Y., Kang, Y.-Y., Yang, H. & Cai, W.-B. Boron-Doped Palladium Nanoparticles on Carbon Black as a Superior Catalyst for Formic Acid Electro-oxidation. J. Phys.Chem. C 113, 8366–8372 (2009).
Beck, M., Ellner, M. & Mittemeijer, E. J. inEuropean Powder Diffraction, Pts 1 and 2 Vol. 321-3 Materials Science Forumeds (eds Delhez R., Mittemeijer E. J. 604–609Trans Tech Publications Ltd (2000).
Ziemecki, S. B., Jones, G. A., Swartzfager, D. G., Harlow, R. L. & Faber, J. Formation of interstitial palladium-carbon phase by interaction of ethylene, acetylene, and carbon monoxide with palladium. J. Am. Chem. Soc. 107, 4547–4548 (1985).
Zlemecki, S. B. & Jones, G. A. Interstitial carbon in palladium. J. Catal. 95, 621–622 (1985).
Krawczyk, M. & Palczewska, W. A surface study on model Pd(111) catalyst modified with boron. Surf. Sci. 287-288, 212–216 (1993).
Lengeler, B. EXAFS study of the structure of boron in palladium. Solid State Commun. 55, 679–682 (1985).
Liu, H. et al. An excellent Pd-based nanocomposite catalyst for the selective hydrogenation of para-chloronitrobenzene. J. Mol. Catal. A: Chem. 308, 79–86 (2009).
Liang, M., Wang, X., Liu, H., Liu, H. & Wang, Y. Excellent catalytic properties over nanocomposite catalysts for selective hydrogenation of halonitrobenzenes. J. Catal. 255, 335–342 (2008).
Sreedhar, B., Devi, D. K. & Yada, D. Selective hydrogenation of nitroarenes using gum acacia supported Pt colloid an effective reusable catalyst in aqueous medium. Catal. Commun. 12, 1009–1014 (2011).
Xu, K. et al. Convenient and selective hydrogenation of nitro aromatics with a platinum nanocatalyst under ambient pressure. Adv. Synth. Catal. 353, 1260–1264 (2011).
Yu, Z., Liao, S., Xu, Y., Yang, B. & Yu, D. A remarkable synergic effect of polymer-anchored bimetallic palladium-ruthenium catalysts in the selective hydrogenation of p-chloronitrobenzene. J. Chem. Soc. Chem. Commun. 1155–1156 (1995).
Yang, X., Liu, H. & Zhong, H. Hydrogenation of o-chloronitrobenzene over polymer-stabilized palladium-platinum bimetallic colloidal clusters. J. Mol. Catal. A: Chem. 147, 55–62 (1999).
Tu, W., Cao, S., Yang, L. & Wang, W. Modification effects of magnetic supports and bimetallic structures on palladium nanocluster catalysts. Chem. Eng. J. 143, 244–248 (2008).
Raja, R. et al. Highly efficient catalysts for the hydrogenation of nitro-substituted aromatics. Chem. Commun. 2026–2028 (2005).
Xu, Q., Liu, X.-M., Chen, J.-R., Li, R.-X. & Li, X.-J. Modification mechanism of Sn4+ for hydrogenation of p-chloronitrobenzene over PVP-Pd/[gamma]-Al2O3. J Mol. Catal. A: Chem. 260, 299–305 (2006).
Roelofs, J. C. A. A. & Berben, P. H. First example of high loaded polymer-stabilized nanoclusters immobilized on hydrotalcite: effects in alkyne hydrogenation. Chem. Commun. 970–971 (2004).
Caga, I. T., Shutt, E. & Winterbottom, J. M. The composition of reduced palladium oxide and its behavior as a catalyst for liquid phase hydrogenation. J. Catal. 44, 271–280 (1976).
Krawczyk, M., Sobczak, J. & Palczewska, W. Surface chemistry and catalysis studies on the palladium-boron system in the semihydrogenation of alkynes. Catal. Lett. 17, 21–28 (1993).
Nilsson, A., Pettersson, L. & Norskov, J. Chemical Bonding at Surfaces and Interfaces 532Elsevier (2007).
Burch, R. & Lewis, F. A. Absorption of hydrogen by palladium+boron and palladium+silver+boron alloys. Trans. Faraday Soc. 66, 727–735 (1970).
Studt, F. et al. Identification of non-precious metal alloy catalysts for selective hydrogenation of acetylene. Science 320, 1320–1322 (2008).
Acknowledgements
We thank the EPSRC, UK and Johnson Matthey for funding this work and a DPhil studentship to ACWAC. We also thank Miss Elisabeth Wolf (Oxford Chemistry) for the assistance in the experiments of acetylene hydrogenation. E.C.C. thanks the European Commission for fellowship (PIIF-GA-2009-252242: NANOTUNE). EXAFS experiments were undertaken at the Diamond Light Source (experiment SM-6693-1). G.J. is indebted to the Royal Society for an Industrial Fellowship and a membership of the UK's HPC Materials Chemistry Consortium (EP/F067496). We acknowledge the support from HECToR, the UK's national high-performance computing service provided by UoE HPCx Ltd at the University of Edinburgh, Cray Inc and NAG Ltd.
Author information
Authors and Affiliations
Contributions
C.W.A.C., A.H.M., M.M.-J.L. and C.M.B. contributed to material synthesis, testing and characterization and E.C.C. and J.C. contributed to EXAFS analysis, W.C.H.K. contributed to microscopic and EDX analysis, C.T. contributed to synchrotron XRD characterization; G.J. contributed to DFT modelling; S.C.E.T. initiated, supervised and coordinated the research. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Figures 1-20, Supplementary Tables 1-8, Supplementary Notes, Supplementary Discussion, Supplementary Methods and Supplementary References. (PDF 1549 kb)
Rights and permissions
About this article
Cite this article
Chan, C., Mahadi, A., Li, MJ. et al. Interstitial modification of palladium nanoparticles with boron atoms as a green catalyst for selective hydrogenation. Nat Commun 5, 5787 (2014). https://doi.org/10.1038/ncomms6787
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/ncomms6787
This article is cited by
-
B-modified Pd/ZnAl2O4 Catalyst for Enhancing CO2 Hydrogenation to Methanol
Catalysis Letters (2024)
-
Atomically dispersed materials: Ideal catalysts in atomic era
Nano Research (2023)
-
Tuning Active Metal Atomic Spacing by Filling of Light Atoms and Resulting Reversed Hydrogen Adsorption-Distance Relationship for Efficient Catalysis
Nano-Micro Letters (2023)
-
High-entropy Metal Oxide-polyoxometalate-palladium Sub-1 nm Nanowires for Semi-hydrogenation Reaction of Alkynes
Chemical Research in Chinese Universities (2023)
-
Interstitial boron-triggered electron-deficient Os aerogels for enhanced pH-universal hydrogen evolution
Nature Communications (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.