Abstract
Amines are a fundamentally important class of biologically active compounds and the ability to manipulate their physicochemical properties through the introduction of fluorine is of paramount importance in medicinal chemistry. Current synthesis methods for the construction of fluorinated amines rely on air and moisture sensitive reagents that require special handling or harsh reductants that limit functionality. Here we report practical, catalyst-free, reductive trifluoroethylation reactions of free amines exhibiting remarkable functional group tolerance. The reactions proceed in conventional glassware without rigorous exclusion of either moisture or oxygen, and use trifluoroacetic acid as a stable and inexpensive fluorine source. The new methods provide access to a wide range of medicinally relevant functionalized tertiary β-fluoroalkylamine cores, either through direct trifluoroethylation of secondary amines or via a three-component coupling of primary amines, aldehydes and trifluoroacetic acid. A reduction of in situ-generated silyl ester species is proposed to account for the reductive selectivity observed.
Similar content being viewed by others
Introduction
The incorporation of fluorine into potential medicines can be used to modify conformation, basicity, intrinsic potency, membrane permeability and pharmacokinetic properties1,2. For these reasons fluorinated entities are particularly important within drug discovery and development3,4. However, the use of fluorine in this field is currently limited to a small number of chemotypes with aryl fluorides being dominant5,6,7,8,9. Given the beneficial properties of fluorine, practical new methods that allow access to a more diverse range of medicinally relevant fluorinated building blocks are of particular importance10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26. For example, β-fluoroalkylamines are less basic than their hydrocarbon counterparts (pKaH 10.7 versus 5.7 for ethylamine and trifluoroethylamine respectively)27 and often exhibit decreased acute toxicity and increased metabolic stability rendering them very attractive in pharmaceutical contexts (for examples, see Fig. 1b)28,29,30,31,32. Unfortunately, while many C–H trifluoroethylation methods have been reported33,34,35,36,37,38, there are fewer reagents for the trifluoroethylation of amines (Fig. 1a, left)39,40. The most widely used method involves LiAlH4 or borane reduction of trifluoromethylamides generated using trifluoroacetic anhydride (Fig. 1a)41,42. The use of pyrophoric reductants requires special precautions, limits applicability on-scale and precludes substrates that contain other reducible functional groups. Alternatively, highly reactive trifluoroacetaldehyde (b.p. −18 °C) or derivatives can be used under milder reductive conditions43. Recent work by Hartwig and co-workers describes palladium-catalysed N-arylation reactions of fluoroalkylamines44. While this opens up an important new synthesis route the approach is inherently limited to fluoroalkylanilines.
Herein we report a practical and catalyst-free method to access structurally diverse β-fluoroalkylamines using trifluoroacetic acid (TFA), which occurs through reduction of in situ-generated silyl ester intermediates.
Results
Reaction design
Seeking to develop a practical method for the fluoroalkylation of free amines, the direct use of TFA was attractive (Fig. 2), owing to its availability, low cost and stability. To the best of our knowledge reductive fluoroalkylation reactions using TFA have only been carried out in the presence of either platinum45 or borane46 catalysts under Schlenk conditions. Therefore, the development of a general and practical method with wide functional group tolerance would constitute a powerful new approach to the synthesis of fluorinated amines.
Trifluoroethylation reactions of secondary amines
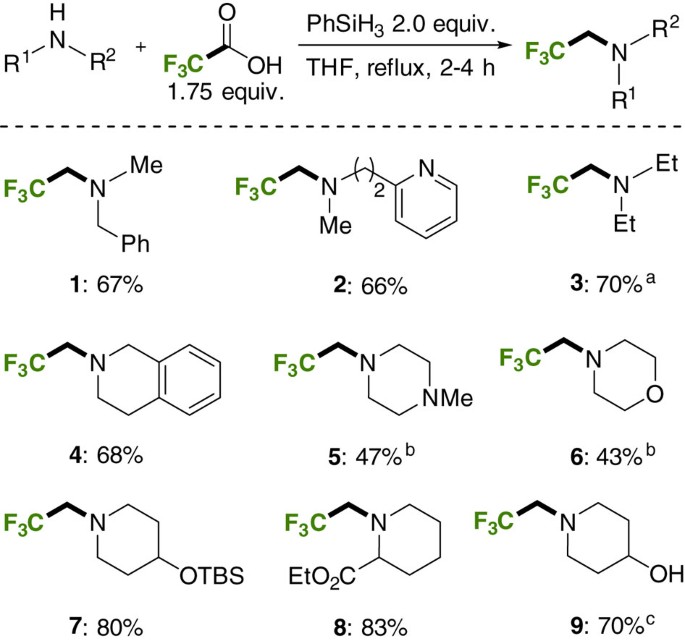
Trifluoroethylation reactions were performed in tetrahydrofuran, with 1.75 equivalents of TFA providing optimal results (Fig. 3; for optimization, see Supplementary Table 1). Importantly, the reactions were carried out in conventional laboratory glassware. Performing the reaction open to air in non-anhydrous solvent was not detrimental (Fig. 3; 1) highlighting the practicality of the method. The phenylsilane and TFA were used as supplied without purification. Acyclic and cyclic secondary amines could be trifluoroethylated in good to moderate isolated yields following flash column chromatography (conversions of 70–90% are typical). Additional moderately basic nitrogen atoms (2, 5) are tolerated as are silyl-protected alcohols (7) and free alcohols (9). In the case of the latter, the hydroxyl group undergoes silylation under the reaction conditions and the silyl group is cleaved upon workup with aqueous base. Most significantly, esters are not reduced under the reaction conditions (8). This transformation would not be possible using the conventional amidation/reduction protocol using LiAlH4 (ref. 43); nor could it be achieved using the Pt45 or borane-catalyzed reactions46. Anilines were relatively poor substrates, with N-methyl anilines typically giving less than 40% conversion to the desired amine (not shown).
Three component trifluoroethylation reactions
We anticipated that we might be able to exploit the acidic, reductive conditions to perform a reductive amination of an aldehyde and a primary amine to form a secondary amine (Fig. 4; brackets), prior to the trifluoroethylation reaction. In this case, a complex, bespoke, β-fluorinated amine core could be generated. As before, these three-component couplings could be carried out in conventional laboratory glassware with moisture exclusion providing small yield gains. The functional group tolerance of this catalyst-free process allows the inclusion of reductively labile, but synthetically valuable, functional groups including alkenes (Fig. 4; 15, 21, 25, 27, 30, 31, 34), esters (16, 26, 39), nitro (11), nitrile (12), Boc-protected amines (33), amides (34), azides (see Supplementary Discussion) and aryl bromides (18, 26, 28, 29, 33, 35). Acid-sensitive functional groups, for example, furans are also tolerated (Fig. 4; 27, 28). The stereo-integrity of amino acid derivative 26 was preserved. Alkyl chloride 22 was prepared at gram-scale (5.00 mmol) from the HCl salt of the amine. The procedure was also amenable to ketones (36–39).
aTypically, amine (0.50 mmol) and aldehyde (0.50 mmol) were mixed neat, before adding toluene (0.5 ml) and PhSiH3 (0.25 mmol) and stirring for 10 min at 70 °C. Trifluoroacetic acid (0.875 mmol) and further PhSiH3 (2.0 mmol) were added and the reaction heated at 70 °C for 16 h. The products were purified by concentration, washing an ether solution of the crude material with sodium bicarbonate and chromatography. Slight timing/temperature modifications are described in Supplementary Methods for: bMethod B (alkyl aldehydes), cMethod C/D (hindered aldehydes), dMethod E (ketones). e5.00 mmol scale from HCl salt of amine, ftrifluoroacetic acid (1.50 mmol) used. gtrifluoroacetic acid (1.00 mmol) used, has mixture of 93:7 trans:cis alkenes.
Typically, an initial imine-forming period was followed by the standard reaction, with minor timing/temperature adjustments beneficial for difficult substrates (full details in Supplementary Methods). Alkyl aldehydes (20, 23, 32, 34) gave poorer conversions relative to stabilized analogues. Slightly increased TFA excesses were beneficial in some cases (27–29). Analysis of the reaction mixtures in these cases revealed competing over-alkylation (double aldehyde addition to the primary amine), with the resulting basic tertiary amines acting to neutralize the required excess acidity. Other competing pathways include amidation of both the initial and alkylated amine.
Mechanistic investigation
The results depicted in Figs 3 and 4 demonstrate that a general trifluoroethylation reaction with wide functional group tolerance is viable in the absence of a catalyst. To understand the process further and account for the remarkable functional group tolerance, we conducted mechanistic experiments to ascertain the provenance of the amine product.
Intuitively, the product might be expected to arise from silane-mediated amidation47, followed by amide reduction by an activated silane; however, an activated reductant is not consistent with the high functional group tolerance observed.
Indeed, trifluoroethylation of piperidine in the presence of trifluoroacetamide 40 resulted in the formation of trifluoroethylamine 41 and complete recovery of amide 40 (Fig. 5a). This result undermines the intervention of an activated reductant/trifluoroacetamide intermediate and implies a separate trifluoroethylation pathway. Further evidence for a discrete amination pathway was obtained when the stoichiometry of TFA to amine was examined (Fig. 5b). When the amine and TFA are equimolar, amide product 40 was obtained in moderate yield and none of the trifluoroethylated amine 1 was formed. In contrast, increasing the amount of TFA to 1.75 equivalents resulted in 80% of the fluorinated amine 1 and only 6% of amide 40. We next examined a series of carboxylic acids and observed that the product ratio varied as a function of pKa (Fig. 5c), with higher acidities leading to more amine. Synthetically useful amounts of alkylated amines, for which there are few convenient preparative procedures, are produced when other chlorine and fluorine-containing acids are employed (Fig. 5c, 42–44).
(a) Trifluoroethylation does not occur via an amide intermediate. (b) Acid stoichiometry controls product distribution. (c) Product distribution varies as a function of acid pKa/electrophilicity. (d) 1H-nuclear magnetic resonance (1H-NMR) experiments demonstrate the rapid generation of silyl esters followed by partial reduction to silyl acetal species. (e) Competition experiment with benzoic acid.
Having ruled out amide reduction we next considered the possible in situ reduction of TFA (or a derivative) to trifluoroacetaldehyde. Given the very high reactivity of this aldehyde it is reasonable to assume that it would react rapidly under the reaction conditions and, in practice, we were unable to observe the aldehyde or derivatives during trifluoroethylation reactions. However, we could identify plausible intermediates via 1H NMR when a typical amine was replaced with substoichiometric (20 mol%) triethylamine (Fig. 5d), which cannot participate in either the amination or amidation reaction. Under these conditions, we observed that phenylsilane reacts with TFA to afford a mixture of silyl ester intermediates (Fig. 5d). No aldehyde was detectable at this point. Over time, the mixture became increasingly complex, due to the ability of each silicon centre to form up to three different Si–O bonds, and for polymeric hydridosiloxanes to form. After standing at room temperature for 24 h, several species derived from trifluoroacetaldehyde were visible including silyl acetals (1H δ 5.10–5.70 3JFH=4.2 Hz) and trifluoroethanol (1H δ 3.98 3JFH=8.9 Hz). Trifluoroacetaldehyde (1H δ 9.43 3JFH=3.5 Hz) was itself also present in smaller amounts. These observations are consistent with silane-mediated reduction of the highly electrophilic TFA-derived silyl ester intermediates. The privileged reactivity of these trifluoroacetoxy silyl esters towards reduction is further apparent from the competition reaction depicted in Fig. 5e. In this case both phenyl and trifluoromethyl-containing silyl esters would be produced leading to amide and amine products respectively.
Based upon these data we depict in Fig. 6 a plausible pathway for trifluoroethylation that is congruous with the foregoing experiments. Specifically, we propose that amine-catalysed dehydrogenative silyl ester formation occurs to generate a mixture of silyl esters, from which point it is possible to generate either amide or amine products depending on the stoichiometry of TFA. In the presence of excess acid (and, therefore, low amine concentration) silane-mediated reduction of the silyl ester intermediates occurs to afford silyl acetals and hemiacetals. Related species at this oxidation level are known to participate in reductive amination reactions with amines in the presence of acetic acid and borohydride or borane reductants43. Under the acidic conditions, an equilibrium43 of the silyl acetals with the amine to form highly reactive iminium ions is proposed. Silane-mediated reduction of the iminium species generates the observed trifluoroethylated products. Conversely, when one equivalent of TFA is used, the concentration of free amine is higher and the electrophilic silyl ester intermediates function as activated acids leading to amide products.
Discussion
In summary, we have demonstrated the first catalyst-free trifluoroethylation reactions of amines using TFA as a bench stable and inexpensive fluorine source. The reactions proceed in conventional glassware without rigorous exclusion of either moisture or air. A wide range of functionalized tertiary β-fluoroalkylamines has been prepared either through direct trifluoroethylation of secondary amines or via a three-component coupling of primary amines, aldehydes and TFA. Importantly, the reaction manifold is applicable to other carboxylic acids, opening the door to an extended range of useful alkylated amines. This novel catalyst-free reaction is expected to be a powerful tool for the construction of functionalized tertiary β-fluoroalkylamines, thereby expanding the range of fluorinated organic compounds for applications in drug discovery, agrochemistry, catalysis and materials science.
Methods
General methods
See Supplementary Methods for further details, and Figs 1–136 for additional data, supporting experiments and spectra.
General procedure for trifluoroethylation of secondary amines
To an oven-dried 10 ml round-bottomed flask fitted with a water condenser under an argon atmosphere (balloon) was added tetrahydrofuran (0.5 ml) and the amine (0.50 mmol) as the free base. The reaction flask was heated in an oil bath at 70 °C and added immediately by microsyringe was phenylsilane (123 μl, 1.00 mmol) followed by TFA (67.0 μl, 0.875 mmol). The reaction was stirred at reflux for 2–4 h, allowed to cool and was concentrated. After dilution with diethyl ether, the material was washed with saturated aqueous sodium bicarbonate, the organics dried over magnesium sulfate and the material purified by flash column chromatography to give the trifluoroethylated amine product.
General procedure for three-component trifluoroethylation reactions
To an oven-dried 10 ml round-bottomed flask fitted with a water condenser under an argon atmosphere (balloon) was added the amine (0.50 mmol) and aldehyde (0.50 mmol). Toluene was added (0.5 ml), followed by phenylsilane (31 μl, 0.25 mmol). The reaction was stirred at 70 °C for 10 min. Then TFA (67.0 μl, 0.875 mmol) and further PhSiH3 (123 μl, 1.00 mmol) were added and the reaction heated at 70 °C for 16 h, and processed as above to yield the trifluoroethylated amine.
Data availability
All data supporting the findings of this study are available within the article and its Supplementary Information file or from the authors on reasonable request. For NMR spectra of the compounds in this article, see Supplementary Figs 11–136.
Additional information
How to cite this article: Andrews, K. G. et al. A practical and catalyst-free trifluoroethylation reaction of amines using trifluoroacetic acid. Nat. Commun. 8, 15913 doi: 10.1038/ncomms15913 (2017).
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
O’Hagan, D. Understanding organofluorine chemistry. An introduction to the C–F bond. Chem. Soc. Rev. 37, 308–319 (2008).
Gillis, E. P., Eastman, K. J., Hill, M. D., Donnelly, D. J. & Meanwell, N. A. Applications of fluorine in medicinal chemistry. J. Med. Chem. 58, 8315–8359 (2015).
Swallow, S. Fluorine in medicinal chemistry. Prog. Med. Chem. 54, 65–133 (2015).
Müller, K., Faeh, C. & Diederich, F. Fluorine in pharmaceuticals: looking beyond intuition. Science 317, 1881–1886 (2007).
Zhou, Y. et al. Next generation of fluorine-containing pharmaceuticals, compounds currently in phase II–III clinical trials of major pharmaceutical companies: new structural trends and therapeutic areas. Chem. Rev. 116, 422–518 (2016).
Campbell, M. G. & Ritter, T. Modern carbon-fluorine bond forming reactions for aryl fluoride synthesis. Chem. Rev. 115, 612–633 (2015).
Brown, J. M. & Gouverneur, V. Transition-metal-mediated reactions for csp2-f bond construction: the state of play. Angew. Chemie Int. Ed. 48, 8610–8614 (2009).
Furuya, T., Kamlet, A. S. & Ritter, T. Catalysis for fluorination and trifluoromethylation. Nature 473, 470–477 (2011).
Hollingworth, C. & Gouverneur, V. Transition metal catalysis and nucleophilic fluorination. Chem. Commun. (Camb) 48, 2929–2942 (2012).
Ventre, S., Petronijevic, F. R. & Macmillan, D. W. C. Decarboxylative fluorination of aliphatic carboxylic acids via photoredox catalysis. J. Am. Chem. Soc. 137, 5654–5657 (2015).
He, Y., Yang, Z., Thornbury, R. T. & Toste, F. D. Palladium-catalyzed enantioselective 1,1-fluoroarylation of aminoalkenes. J. Am. Chem. Soc. 137, 12207–12210 (2015).
Nielsen, M. K., Ugaz, C. R., Li, W. & Doyle, A. G. PyFluor: a low-cost, stable, and selective deoxyfluorination reagent. J. Am. Chem. Soc. 137, 9571–9574 (2015).
Beatty, J. W., Douglas, J. J., Cole, K. P. & Stephenson, C. R. J. A scalable and operationally simple radical trifluoromethylation. Nat. Commun. 6, 7919 (2015).
Fujimoto, T. & Ritter, T. PhenoFluorMix: practical chemoselective deoxyfluorination of phenols. Org. Lett. 17, 544–547 (2015).
Sather, A. C. et al. A fluorinated ligand enables room-temperature and regioselective Pd-catalyzed fluorination of aryl triflates and bromides. J. Am. Chem. Soc. 137, 13433–13438 (2015).
Nagib, D. A. & MacMillan, D. W. C. Trifluoromethylation of arenes and heteroarenes by means of photoredox catalysis. Nature 480, 224–228 (2011).
Liu, H. et al. Ultrafast click chemistry with fluorosydnones. Angew. Chemie Int. Ed. 55, 12073–12077 (2016).
Nahra, F. et al. Hydrofluorination of alkynes catalysed by gold bifluorides. ChemCatChem. 7, 240–244 (2015).
Keddie, N. S., Slawin, A. M. Z., Lebl, T., Philp, D. & O’Hagan, D. All-cis 1,2,3,4,5,6-hexafluorocyclohexane is a facially polarized cyclohexane. Nat. Chem. 7, 483–488 (2015).
Morandi, B. & Carreira, E. M. Expedient preparation of trifluoromethyl-substituted benzofuranols. Org. Lett. 13, 5984–5985 (2011).
Künzi, S. A., Morandi, B. & Carreira, E. M. Preparation of trifluoromethyl-substituted aziridines with in situ generated CF3CHN2 . Org. Lett. 14, 1900–1901 (2012).
Mormino, M. G., Fier, P. S. & Hartwig, J. F. Copper-mediated per fluoroalkylation of heteroaryl bromides with (phen)CuRF. Org. Lett. 16, 1744–1747 (2014).
Arlow, S. I. & Hartwig, J. F. Synthesis of aryldifluoroamides by copper-catalyzed cross-coupling. Angew. Chemie Int. Ed. 55, 4567–4572 (2016).
Banik, S. M., Medley, J. W. & Jacobsen, E. N. Catalytic, asymmetric difluorination of alkenes to generate difluoromethylated stereocenters. Science 353, 51–54 (2016).
Williams, T. J. & Greaney, M. F. Mild chlorodifluoroacylation of indoles via self-activation of sodium chlorodifluoroacetate. Org. Lett. 16, 4024–4027 (2014).
Molnár, I. G. & Gilmour, R. Catalytic difluorination of olefins. J. Am. Chem. Soc. 138, 5004–5007 (2016).
Morgenthaler, M. et al. Predicting and tuning physicochemical properties in lead optimization: amine basicities. ChemMedChem. 2, 1100–1115 (2007).
Vorberg, R. et al. Effect of partially fluorinated N -alkyl-substituted piperidine-2-carboxamides on pharmacologically relevant properties. ChemMedChem. 11, 2216–2239 (2016).
Huchet, Q. A. et al. Fluorination patterning: a study of structural motifs that impact physicochemical properties of relevance to drug discovery. J. Med. Chem. 58, 9041–9060 (2015).
Xu, F. et al. Asymmetric synthesis of telcagepant, a CGRP receptor antagonist for the treatment of migraine. J. Org. Chem. 75, 7829–7841 (2010).
Adams, J. L. et al. Pyrimidinylimidazole inhibitors of p38: cyclic N-1 imidazole substituents enhance p38 kinase inhibition and oral activity. Bioorg. Med. Chem. Lett. 11, 2867–2870 (2001).
van Oeveren, A. et al. Discovery of 6-N,N -Bis(2,2,2-trifluoroethyl)amino- 4-trifluoromethylquinolin-2(1 H )-one as a novel selective androgen receptor modulator #. J. Med. Chem. 49, 6143–6146 (2006).
Yang, J. et al. Pd-catalyzed divergent trifluoroethylation and arylation of arylboronic acids by aryl(2,2,2-trifluoroethyl)iodonium triflates. Org. Biomol. Chem. 14, 7654–7658 (2016).
Fujiwara, Y. et al. Practical and innate carbon–hydrogen functionalization of heterocycles. Nature 492, 95–99 (2012).
O’Hara, F. et al. Preparation and purification of zinc sulfinate reagents for drug discovery. Nat. Protoc. 8, 1042–1047 (2013).
Tóth, B. L., Kovács, S., Sályi, G. & Novák, Z. Mild and efficient palladium-catalyzed direct trifluoroethylation of aromatic systems by C–H activation. Angew. Chemie Int. Ed. 55, 1988–1992 (2016).
Kreis, L. M., Krautwald, S., Pfeiffer, N., Martin, R. E. & Carreira, E. M. Photocatalytic synthesis of allylic trifluoromethyl substituted styrene derivatives in batch and flow. Org. Lett. 15, 1634–1637 (2013).
Song, W., Lackner, S. & Ackermann, L. Nickel-catalyzed C–H alkylations: direct secondary alkylations and trifluoroethylations of arenes. Angew. Chemie Int. Ed. 53, 2477–2480 (2014).
Luo, H., Wu, G., Zhang, Y. & Wang, J. Silver(I)-catalyzed N -trifluoroethylation of anilines and O-trifluoroethylation of amides with 2,2,2-trifluorodiazoethane. Angew. Chemie Int. Ed. 54, 14503–14507 (2015).
Umemoto, T. & Gotoh, Y. 1,1-dihydroperfluoroalkylations of nucleophiles with (1,1-dihydroperfluoroalkyl)phenyliodonium triflates. J. Fluor. Chem. 31, 231–236 (1986).
Wong, J. C. et al. Pharmacokinetic optimization of class-selective histone deacetylase inhibitors and identification of associated candidate predictive biomarkers of hepatocellular carcinoma tumor response. J. Med. Chem. 55, 8903–8925 (2012).
Kim, H. Y. et al. Exploration of the activity of 7-pyrrolidino-8- methoxyisothiazoloquinolones against methicillin-resistant Staphylococcus aureus (MRSA). J. Med. Chem. 54, 3268–3282 (2011).
Mimura, H., Kawada, K., Yamashita, T., Sakamoto, T. & Kikugawa, Y. Trifluoroacetaldehyde: a useful industrial bulk material for the synthesis of trifluoromethylated amino compounds. J. Fluor. Chem. 131, 477–486 (2010).
Brusoe, A. T. & Hartwig, J. F. Palladium-catalyzed arylation of fluoroalkylamines. J. Am. Chem. Soc. 137, 8460–8468 (2015).
Sorribes, I., Junge, K. & Beller, M. Direct catalytic N-alkylation of amines with carboxylic acids. J. Am. Chem. Soc. 136, 14314–14319 (2014).
Fu, M. C., Shang, R., Cheng, W. M. & Fu, Y. Boron-catalyzed N-alkylation of amines using carboxylic acids. Angew. Chemie Int. Ed. 54, 9042–9046 (2015).
Andrews, K. G., Summers, D. M., Donnelly, L. J. & Denton, R. M. Catalytic reductive N-alkylation of amines using carboxylic acids. Chem. Commun. (Camb) 52, 1855–1858 (2016).
Acknowledgements
The School of Chemistry, University of Nottingham is acknowledged for funding (PhD studentship to K.G.A.).
Author information
Authors and Affiliations
Contributions
R.M.D. and K.G.A. designed the experiments. K.G.A. and R.F. carried out the experiments and collected the data. R.M.D., K.G.A and R.F. analysed the data. R.M.D and K.G.A. drafted the manuscript. All authors approved the final version of the manuscript for submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary figures, supplementary table, supplementary discussion, supplementary methods and supplementary references. (PDF 8769 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Andrews, K., Faizova, R. & Denton, R. A practical and catalyst-free trifluoroethylation reaction of amines using trifluoroacetic acid. Nat Commun 8, 15913 (2017). https://doi.org/10.1038/ncomms15913
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/ncomms15913
This article is cited by
-
Reactivity-based identification of oxygen containing functional groups of chemicals applied as potential classifier in non-target analysis
Scientific Reports (2023)
-
Fluoroalkyl N-sulfonyl hydrazones: An efficient reagent for the synthesis of fluoroalkylated compounds
Science China Chemistry (2021)
-
The plasticity of DNA replication forks in response to clinically relevant genotoxic stress
Nature Reviews Molecular Cell Biology (2020)
-
Use of trifluoroacetaldehyde N-tfsylhydrazone as a trifluorodiazoethane surrogate and its synthetic applications
Nature Communications (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.