Abstract
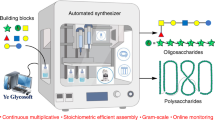
Automated glycan assembly (AGA) has advanced from a concept to a commercial technology that rapidly provides access to diverse oligosaccharide chains as long as 30-mers. To date, AGA was mainly employed to incorporate trans-glycosidic linkages, where C2 participating protecting groups ensure stereoselective couplings. Stereocontrol during the installation of cis-glycosidic linkages cannot rely on C2-participation and anomeric mixtures are typically formed. Here, we demonstrate that oligosaccharides containing multiple cis-glycosidic linkages can be prepared efficiently by AGA using monosaccharide building blocks equipped with remote participating protecting groups. The concept is illustrated by the automated syntheses of biologically relevant oligosaccharides bearing various cis-galactosidic and cis-glucosidic linkages. This work provides further proof that AGA facilitates the synthesis of complex oligosaccharides with multiple cis-linkages and other biologically important oligosaccharides.
Similar content being viewed by others
Introduction
Carbohydrates are the most abundant biomolecules on earth and serve many functions, including structure, nutrition and information transfer1. The structural diversity and complexity of natural glycans, combined with the lack of amplification and expression methods, renders their isolation difficult and often impossible. The synthesis of complex glycans is time consuming and is performed by specialists. Automated glycan assembly (AGA) on solid support2,3 has accelerated the procurement of defined oligosaccharides for use as molecular tools on glycan arrays4 and as part of vaccines5. Using automated synthesis, oligosaccharides as large as 30-mers6 that represent different subclasses of glycans, including glycolipids7 and glycosaminoglycans8, are now accessible. AGA utilizes monomeric building blocks that are combined on a synthesizer executing a set of preprogrammed commands that are combined into modules9. To date, a set of monosaccharide building blocks has been identified for the efficient and reliable incorporation of trans-glycosidic linkages during AGA. These monomeric building blocks commonly take advantage of a C2 neighbouring group participation to ensure exclusive trans-glycosylations. Most oligosaccharides assembled by AGA to date contained either exclusively or predominantly trans-glycosidic linkages aside from few exceptions10,11. The selective formation of a 1,2-cis-glycosylic linkage during AGA of the tumour-associated hexasaccharide antigen Globo-H10, as well as a β-(1,4)-mannuronic acid alginate 12-mer containing 1,2-cis-mannosides11 relied on C2 non-participating protecting groups and benefited from leaving group effects10 and conformational influences11.
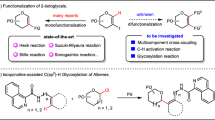
Stereoselective formation of 1,2-cis-glycosidic linkages still remains challenging12,13,14,15,16, because non-participating C2 protecting groups generally lead to mixtures of stereoisomers that have to be separated at the end of the synthesis (Fig. 1a). Various strategies for the stereoselective synthesis of 1,2-cis-glycosides have been described (Fig. 1). Intramolecular aglycon delivery (IAD)12, where the nucleophile is transferred from the adjacent C2 carbon to the anomeric position, is technically challenging when more than one cis-linkage is to be created. Hydrogen-bond-mediated aglycan delivery (HAD) method showed that installing picolinyl and picoloyl protecting groups on the C6 position is powerful for the synthesis of cis-glucosidic linkage, but fails for cis-galactosidic linkages13, as none of the hydroxyl groups at C3, C4 or C6 faces towards the bottom of the ring. Chiral auxiliaries at C2 provide selectivity14, but require two additional steps to be removed. Cleavage of the chiral auxiliary may result in a loss of benzyl ethers during solid-phase synthesis15. Additives can improve stereoselectivity by forming a less reactive intermediate in situ 16 but are hard to use during automated syntheses. Remote participation by protecting groups placed at the C3, C4 and/or C6 positions of glucose (Glc) and galactose (Gal) building blocks can control the stereoselectivity of glycosylations17,18,19. Building blocks containing common remote participating groups are attractive for automated synthesis as they fit the coupling–deprotection scheme and require no additional manipulations.
(a) A non-participating protecting group at C2 results in a mixture of anomers. (b) Intramolecular aglycon delivery with a C2 2-naphthylmethyl (NAP)-ether produces predominantly the β-anomer. (c) Hydrogen-bond-mediated aglycan delivery using picolinyl and picoloyl protecting groups at the C6 position of glucose provide high selectivity. (d) Chiral auxiliaries ensure complete selectivity. (e) Additive (or solvent)-modulation results in predominant to exclusive formation of 1,2-cis-glycosides. (f) Remote participating groups result in good selectivities. Ac, acetate; Bn, benzyl; DMF, dimethylformamide; LG, leaving group; Nap, naphthyl; [O], oxidation; PG, protecting group; Pico, picoloyl; ROH, acceptor; Ph, phenyl.
Here, we demonstrate that AGA of oligosaccharides containing 1,2-cis-glycosidic linkages is feasible when monosaccharide building blocks containing common remote participating groups are used. This work proves that identification and incorporation of reliable building blocks into AGA protocols provides accessibility to biologically relevant oligosaccharides containing cis-linkages.
Results
Selection of oligosaccharides bearing 1,2-cis glycosidic linkages
To expand the scope of AGA to the stereoselective installation of cis-glycosidic linkages, we focused on cis-glucosides and cis-galactosides as the most prevalent 1,2-cis-linkages in mammalian and bacterial glycomes20,21. Oligosaccharides (1–4) bearing α-galactosidic linkages and α-glucans examples (5–9) were selected as targets to develop automated methods for stereoselective cis-glycosidic bond formation (Fig. 2).
AGA of oligosaccharides containing 1,2-cis-glactosidic linkage
Since the stereo- and regiochemical information for glycosidic bond formation mainly resides in the building blocks9, the synthetic considerations focused initially on the identification of monomers containing remote participating groups. α-Gal trisaccharide epitope 1 (ref. 22) served as a model system to optimize the formation of α-(1,3) galactosides. The influence of remote participation on the stereochemical outcome of AGA couplings was explored using seven thiogalactoside building blocks (13a–13g) containing acetyl or benzoyl esters (Ac, Bz). Solid-support-bound disaccharide 14 was assembled using building blocks 11 and 12 on a polystyrene resin equipped with a photocleavable linker 10 (ref. 8). Disaccharide 14 served as the acceptor in glycosylations employing monomers 13a–13g. Incorporation of each building block was achieved by the addition of twice five equivalents monomer that was activated at activation temperature Ta (−40 °C) for 5 min (t1) by the addition of a N-iodosuccinimide (NIS)/Trifluoromethanesulfonic acid (TfOH) activator solution, followed by incubation at the incubation temperature Ti (−20 °C) for 25 min (t2). Following ultraviolet cleavage from the polystyrene resin, trisaccharides (15a–15g) were analysed by normal-phase high-performance liquid chromatography (NP-HPLC) (Table 1) to determine conversion and stereoselectivity of the glycosylation reaction.
Galactose building block 13a (ref. 23), protected only with non-participating benzyl ether groups, relies exclusively on the anomeric effect to drive α-galactoside formation23 and produced mainly the desired anomer 15a (ref. 24) (α:β=13.8:1) (entry 1). Thiogalactosides 13b (ref. 25) and 13c bearing C6 acetyl or benzoyl esters resulted in lower selectivity (α:β=6.4−5.8:1) (entries 2 and 3). These results are consistent with reports that C6 esters decrease the α-selectivity of galactose18. The presence of a C4-acetyl or benzoyl ester in thiogalactosides 13d and 13e drastically improved the α-selectivity (26.5−26.2:1). Expanding on the C4-ester effect, a second ester was placed on thiogalactose building blocks. Glycosylation of 14 with C3-, C4-bis-acetylated thiogalactose 13f proceeded with drastically improved selectivity (39.5:1) (Table 1, entry 6). Building block 13g, carrying C4 and C6 esters proceeded with complete stereoselectivity but significant amounts of the disaccharide deletion sequence remained after the coupling (entry 7). This study proved that the 1,2-cis-galactosidic linkage can be installed by using building blocks that take advantage of remote participation groups but require no additives or chiral auxiliaries.
With optimized building blocks in hand (Fig. 3a), the α-Gal epitope pentasaccharide 2 was prepared by AGA (Fig. 3b). All synthetic manipulations were executed on an automated oligosaccharide synthesizer working through preprogrammed steps that were combined into modules for each synthetic transformation. An initial acidic trimethylsilyl trifluoromethanesulfonate (TMSOTf) wash neutralizes basic residues that accumulate during dimethylformamide (DMF) washes and removes any water present in the synthesizer. Glycosylation with twice five equivalents monomer was followed by fluorenylmethyloxycarbonyl (Fmoc) removal by treatment with a solution of triethylamine (TEA) in DMF (v/v, 1/4). The glycosylation efficiency was estimated by quantitating the ultraviolet absorption of the released dibenzofulvene. Following automated assembly of the fully protected oligosaccharide targets, cleavage from the resin by ultraviolet irradiation was carried out using a continuous-flow photoreactor8. Purification by preparative NP-HPLC provided pentasaccharide 15f (41% based on the resin loading over ten steps on resin) and 23 (33% over ten steps on resin). The conjugation-ready α-Gal epitope trisaccharide 1 (48% over two steps) and pentasaccharide 2 (48%) were purified by reverse-phase HPLC following deprotection of 15f and 23 by methanolysis and hydrogenolysis.
(a) Resin functionalized with the photolabile linker (10) and monosaccharide building blocks. (b) Automated assembly and post-automation steps yield conjugation-ready oligosaccharides 1–4. For details, see Supplementary Tables 3–5.
Building blocks carrying remote participation groups were employed in AGA of Globo-series oligosaccharides that contain a Gal-α-(1,4)-Gal-β-(1,4)-Glc common trisaccharide core (3) (refs 26, 27). Globo-H (4), a hexasaccharide vaccine candidate currently in advanced clinical trials28,29 contains the core trisaccharide 3. Thiogalactoside building block 19 was designed based on thiogalactoside 13f to allow for C3 elongation en route to Globo-H and to benefit from remote participation. The influence of a remote C4 participation group present in monomer 19 was studied in the context of the AGA of trisaccharides 24 (ref. 24) and 25. Trisaccharide 25 using optimized building block 19 was obtained in 41% yield, and with excellent selectivity (α:β=19:1), while 24 using perbenzylated thiogalactoside donor 13a was isolated in 35% yield and moderate selectivity (α:β=4.3:1). This drastic improvement of stereoselective 1,2-cis-galactosidic bond formation emphasizes the importance of the remote participating group on the C4 for the efficient synthesis of complex oligosaccharides containing cis-galactoside(s) linkage using AGA. Similarly Globo-H hexasaccharide 4 was prepared by AGA using building blocks 17–22. Fully protected oligosaccharides 25 and 26 were deprotected to furnish 3 (51% yield) and 4 (42%) respectively. The stereoselectivity of α-galactoside formation can dramatically benefit from the use of ‘approved’ building blocks with remote participation.
AGA of α-glucans bearing multiple 1,2-cis-glucosidic linkages
The use of remote participation is not limited to the formation of cis-galactosidic linkages but can be generally employed as illustrated for 1,2-cis-glucosidic linkages19. Eight thioglucoside building blocks (29a–29h) exhibiting various protecting group patterns were synthesized to explore the influence of remote participation. AGA of disaccharides 30a–30j was achieved using thioglycosides 17, 27 and 28, and building blocks 29a–29h before photocleavage from the polystyrene resin yielded disaccharides 30a–30j that were analysed by HPLC (Table 2). Glucose building block 29h with remote participating acetyl groups in the C3 and C6 positions in analogy to the galactose series, resulted in the best selectivity and further benefited from the addition of diethyl ether to promote the desired α-glucoside formation (Table 2).
With reliable glucose building blocks and AGA protocols for the incorporation of α-glucosides in place, a series of α-glucans were prepared. The initial target, pentasaccharide 5 containing four consecutive α-(1→6)-glycosidic linkages15 was selected as proof-of-principle, since it had been prepared previously via the chiral auxiliary approach. AGA of pentasaccharide 5 was achieved by addition of the thioglycoside 27 to the support-bound linker 10 to form the initial β-glucoside linkage. Four successive glycosylation cycles using thioglycoside 31 introduced four consecutive α-1,2-cis-glucosidic linkages. Ultraviolet-induced cleavage of the fully protected oligosaccharide from the resin yielded protected pentasaccharide 34 in 23% over 11 steps on resin using NP-HPLC (Fig. 4b).
(a) Monosaccharide building blocks. (b) AGA of conjugation-ready oligosaccharides 5–9. For details, see Supplementary Tables 8–10.
Pentasaccharide 6, containing multiple α-(1→4) linkages, and 7 with multiple α-(1→3) glucosides were assembled using the corresponding building blocks (Fig. 4). The lower reactivity of the C4-hydroxyl group as glycosyl acceptor was compensated by prolonged glycosylation times (t2=50 min) during AGA of 35 using building blocks 27 and 32 (see Supplementary Table 8). Cleavage from the resin and preparative NP-HPLC yielded 9% of 35 over 11 steps. Using a similar AGA process, pentasaccharide 36 was obtained in 12% yield over 11 steps. The syntheses of 35 and 36 illustrated that glycosylations of secondary C3 or C4 hydroxyl groups are less efficient and less stereoselective than those involving primary C6 hydroxyl groups as nucleophiles. Such differences in glycosylation efficiency are well known for solution phase strategies. It is anticipated that the glycosylation modules (for example, number of glycosylation, reaction time or temperature) upon AGA will improve glycosylation efficiency and seteroselectivity. Removal of the protective groups from pentasaccharides 34–36 yielded conjugation-ready pentasaccharides 5 (47% yield), 6 (33%) and 7 (31%) following reverse-phase HPLC.
Two naturally occurring oligosaccharide fragments bearing multiple 1,2-cis-glucosidic linkages were assembled using the building blocks and protocols established above. The immune-modulatory pentasaccharide 8 (ref. 30), containing α-(1→3) and α-(1→6) glucosidic linkages, and tetrasaccharides 9 (ref. 31), known for activating toll-like receptor containing α-(1→4) and α-(1→6) glucosides, were synthesized. Deprotection of oligosaccharide 37 that was assembled in 17% yield over 10 steps and 38 yielding 20% over eight steps, furnished oligosaccharides 8 (47% yield) and 9 (33%).
In conclusion, we demonstrate that building blocks bearing remote participating groups are effective in assembling oligosaccharides containing cis-glucosidic and cis-galactosidic linkages. Nine oligosaccharides (1–9) were assembled to illustrate the power of the AGA approach. Standardized synthesis as well as deprotection and purification protocols enabled us to procure conjugation-ready molecules. The reliable incorporation of cis-gluco- and cis-galactosides into oligosaccharides by automated synthesis complements earlier successes in installing trans-glycosides, to render AGA the method of choice for the procurement of complex glycans.
Methods
General
The synthesis of characterization of monomer building blocks (11, 12, 13a–13g, 16–22, 27, 28, 29a–29h and 31–33) and the synthetic protocols including liquid chromatography (LC)–mass spectrometry (MS) chromatograms for AGA of oligosaccharides (1–9, 15a–15g, 23–26, 30a–30j and 34–38) are provided in Supplementary Methods. For 1H, 13C and two-dimensional (2D) NMR spectra of the compounds in the article, see Supplementary Figs 1–118. For the reaction conditions on AGA, see Supplementary Tables 1,3,6 and 8. For yields for oligosaccharides, see Supplementary Tables 2,4,5,7,9 and 10.
Pre-automation steps
Synthesis of building blocks and the linker-bound resin are described in Supplementary Methods. For preparation of building block solutions, reagent solutions and programs for AGA, see Supplementary Methods.
AGA
The automated synthesizer8 executes a series of commands that are combined into modules to achieve specific transformations (see Supplementary Methods and Supplementary Tables).
Module 1: acid wash
The resin was washed with DMF, tetrahydrofuran (THF) and dichloromethane (DCM) (three times each with 2 ml for 15 s). The resin was swollen in 2 ml DCM, and the temperature of the reaction vessel was adjusted to −20 °C. For an acidic wash, 0.500 ml of the TMSOTf in DCM was delivered to the reaction vessel. After 1 min, the solution was drained. The resin was swollen in 2 ml DCM and the temperature of the reaction vessel was adjusted to the activation temperature (Ta).
Module 2: glycosylation using thioglycosides
During temperature adjustment, the DCM in the reaction vessel was drained and a solution of building block (5.0 equiv. in 1.0 ml DCM) was delivered to the reaction vessel. After the activation temperature (Ta) was reached, the reaction was started by addition of 1.0 ml of NIS solution (5.5 equiv. in 1.0 ml) and TfOH (0.2 equiv. in 1.0 ml) in DCM and dioxane (v/v, 9/1). The glycosylation mixture was activated for an activation time (t1=5 min) at Ta, linearly ramped to incubation temperature (Ti), and finally incubated for an additional incubation time (t2) minutes at Ti. Then, the reaction solution was drained and the resin was washed with DCM (six times with 2 ml for 15 s). This procedure was repeated twice. The values of Ta, t1, Ti and t2 are shown in Supplementary Tables.
Module 3: fmoc deprotection
The resin was washed with DMF (six times with 2 ml for 15 s), swollen in 2 ml DMF and the temperature of the reaction vessel was adjusted to 25 °C. The DMF was drained and 3.5 ml of a solution of TEA in DMF was delivered to the reaction vessel. After 5 min, the reaction solution was collected in the fraction collector of the oligosaccharide synthesizer. This procedure was repeated twice.
Cleavage and purification
Resin cleavage. The FEP tubing of the photoreactor8 was washed with 20 ml DCM at a flow rate of 5 ml min−1 to prepare the reactor. For cleavage, the resin was slowly injected into the reactor using a disposable syringe (20 ml) and pushed through the tubing with 18 ml DCM (flow rate: 600 μl min−1). The tubing is washed with 20 ml DCM (flow rate: 2 ml min−1) to remove any remaining resin. The suspension leaving the reactor was passed through a filter to remove the resin. The system was re-equilibrated by washing the tubes with 20 ml DCM at a flow rate of 5 ml min−1. The cleavage procedure was performed twice and the resulting solution was evaporated before the crude material was analysed by matrix-assisted laser desorption/ionization–time of flight–MS (MALDI–TOF–MS), NMR and HPLC.
Analytical HPLC. The crude material was analysed by HPLC (column: Luna 5μ Sil 100A, (260 × 4.60 mm); flow rate: 1 ml min−1; eluents: hexane/ethyl acetate; gradient: 20% (5 min), 60% (in 40 min), 100% (in 5 min); and detection: evaporative light scattering detector (ELSD)).
Preparative HPLC. The crude mixture was dissolved in a minimum volume of DCM and 0.9 ml of 20% hexane in ethyl acetate and injected for purification using semi-preparative HPLC (column: Luna 5μ Sil (260 × 10 mm); flow rate: 5 ml min−1; eluents: 5% DCM in hexane/5% DCM in ethyl acetate; gradient: 20% (5 min), 60% (in 40 min), 100% (in 5 min); and detection: ELSD) to afford the fully protected target oligosaccharides.
Deprotection conditions. To a solution of the fully protected oligosaccharide in MeOH (5 ml) was added 58 μl of a 0.5 M NaOMe solution (0.25 equiv. per acetyl of benzoyl group) at 40 °C. The mixture was stirred until thin-layer chromatography (TLC) analysis indicated complete deprotection, then neutralized with 200 mg Amberite (400 mg per 100 μl of NaOMe solution). The amberlite was filtered off and the crude filtrate was evaporated and re-dissolved in MeOH, EtOAc and AcOH (v/v/v=5:0.5:0.2) before 5% Pd/C (W/V) was added, purged first with argon and then with hydrogen gas (H2), and was left to stir overnight at room temperature. The reaction mixture was filtered through a syringe filter with 20 ml of a water/methanol mixture (9:1) and the combined solution was evaporated to provide the crude product.
Analysis of oligosaccharide products
Analytical HPLC. The crude material was analysed by HPLC (column: Hypercarb, (150 × 4.60 mm); flow rate: 0.8 ml min−1; eluents: 0.1% FA in acetonitrile/0.1% FA in water; gradient: 0% (10 min), 30% (in 30 min), 100% (in 5 min); and detection: ELSD).
Preparative HPLC. The crude product was purified by preparative HPLC (column: Hypercarb, (150 × 10.00 mm); flow rate: 3.6 ml min−1; eluents: 0.1% FA in acetonitrile/0.1% FA in water; gradient: 0% (10 min), 30% (in 30 min), 100% (in 5 min); and detection: ELSD) to afford the oligosaccharide product.
Data availability
The raw data used to generate the figures and tables in this manuscript are available from the corresponding author on request.
Additional information
How to cite this article: Hahm, H. S. et al. Automated assembly of oligosaccharides containing multiple cis-glycosidic linkages. Nat. Commun. 7:12482 doi: 10.1038/ncomms12482 (2016).
References
Dwek, R. A. Glycobiology: toward understanding the function of sugars. Chem. Rev. 96, 683–720 (1996).
Plante, O. J., Palmacci, E. R. & Seeberger, P. H. Automated solid-phase synthesis of oligosaccharides. Science 291, 1523–1527 (2001).
Seeberger, P. H. The logic of automated glycan assembly. Acc. Chem. Res. 48, 1450–1463 (2015).
Kröck, L. et al. Streamlined access to conjugation-ready glycans by automated synthesis. Chem. Sci. 3, 1617–1622 (2012).
Hewitt, M. C., Snyder, D. A. & Seeberger, P. H. Rapid synthesis of a glycosylphosphatidylinositol-based malaria vaccine using automated solid-phase oligosaccharide synthesis. J. Am. Chem. Soc. 124, 13434–13436 (2002).
Calin, O., Eller, S. & Seeberger, P. H. Automated polysaccharide synthesis: assembly of a 30 mer mannoside. Angew. Chem. Int. Ed. 52, 5862–5865 (2013).
Love, K. R. & Seeberger, P. H. Automated solid-phase synthesis of protected tumor-associated antigen and blood group determinant oligosaccharides. Angew. Chem. Int. Ed. 43, 602–605 (2004).
Eller, S., Collot, M., Yin, J., Hahm, H. S. & Seeberger, P. H. Automated solid-phase synthesis of chondroitin sulfate glycosaminoglycans. Angew. Chem. Int. Ed. 52, 5858–5861 (2013).
Mulani, S. K., Hung, W.-C., Ingle, A. B., Shiau, K.-S. & Mong, K.-K. T. Modulating glycosylation with exogenous nucleophiles: an overview. Org. Biomol. Chem. 12, 1184–1197 (2014).
Werz, D. B., Castagner, B. & Seeberger, P. H. Automated synthesis of the tumor-associated carbohydrate antigens Gb-3 and Globo-H: incorporation of α-galactosidic linkages. J. Am. Chem. Soc. 129, 2770–2771 (2007).
Walvoort, M. T. C. et al. Automated solid-phase synthesis of β-mannuronic acid alginates. Angew. Chem. Int. Ed. 51, 4393–4396 (2012).
Ishiwata, A., Lee, Y. J. & Ito, Y. Recent advances in stereoselective glycosylation through intramolecular aglycon delivery. Org. Biomol. Chem. 8, 3596–3608 (2010).
Yasomanee, J. P. & Demchenko, A. V. Effect of remote picolinyl and picoloyl substituents on the stereoselectivity of chemical glycosylation. J. Am. Chem. Soc. 134, 20097–20102 (2012).
Kim, J. H., Yang, H. & Boons, G. J. Stereoselective glycosylation reactions with chiral auxiliaries. Angew. Chem. Int. Ed. 44, 947–949 (2005).
Boltje, T. J., Kim, J. H., Park, J. & Boons, G. J. Chiral-auxiliary-mediated 1,2-cis-glycosylations for the solid-supported synthesis of a biologically important branched a-glucan. Nat. Chem. 2, 552–557 (2010).
Lu, S.-R., Lai, Y.-H., Chen, J.-H., Liu, C.-Y. & Mong, K.-K. T. Dimethylformamide: an unusual glycosylation modulator. Angew. Chem. Int. Ed. 50, 7315–7320 (2011).
Vidal S., Wertz D. (eds). Modern Synthetic Methods in Carbohydrate Chemistry—From Monosaccharides to Complex Glycoconjugates Wiley (2014).
Li, Z., Zhu, L. & Kalikanda, J. Development of a highly α-selective galactopyranosyl donor based on a rational design. Tetrahedron Lett. 52, 5629–5632 (2011).
Komarova, B. S. et al. Is an acyl group at O-3 in glucosyl donors able to control α-stereoselectivity of glycosylation? The role of conformational mobility and the protecting group at O-6. Carbohydr. Res. 384, 70–86 (2014).
Demchenko A. V. (ed). Handbook of Chemical Glycosylation: advances in Stereoselectivity and Therapeutic Relevance Wiley (2008).
Adibekian, A. et al. Comparative bioinformatics analysis of the mammalian and bacterial glycomes. Chem. Sci. 2, 337–344 (2011).
Galili, U. Interaction of the natural anti-Gal antibody with alpha-galactosyl epitopes: amojor obstacle for xenotransplantation in humans. Immunol. Today 14, 480–482 (1993).
Demchenko, A. V., Rousson, E. & Boons, G. J. Stereoselective 1,2-cis-galactosylation assisted by remote neighboring group participation and solvent effects. Tetrahedron Lett. 40, 6523–6526 (1999).
Hoffman, J., Hahm, H. S., Seeberger, P. H. & Pagel, K. Identification of carbohydrate anomers using ion mobility-mass spectrometry. Nature 526, 241–244 (2015).
Lourenco, E. C. & Ventura, M. R. The synthesis of compatible solute analogues—solvent effects on selective glycosylation. Carbohydr. Res. 346, 163–168 (2011).
Danishefsky, S. J., Shue, Y.-K., Chang, M. N. & Wong, C.-H. Development of Globo-H cancer vaccine. Acc. Chem. Res. 48, 643–652 (2015).
Wang, Z., Zhou, L., El-Boubbou, K., Ye, X.-S. & Huang, X. Multi-component one-pot synthesis of the tumor-associated carbohydrate antigen Globo-H based on preactivation of thioglycosyl donors. J. Org. Chem. 72, 6409–6420 (2007).
Gilewski, T. et al. Immunization of metastatic breast cancer patients with a fully synthetic globo H conjugate: a phase I trial. Proc. Natl Acad. Sci. USA 98, 3270–3275 (2001).
Wilson, R. M. & Danishefsky, S. J. A vision for vaccines built from fully synthetic tumor-associated antigens: from the laboratory to the clinic. J. Am. Chem. Soc. 135, 14462–14472 (2013).
Zhao, C., Li, M., Luo, Y. & Wu, W. Isolation and structural characterization of an immunostimulating polysaccharide from fuzi Aconitum carmichaeli. Carbohydr. Res. 341, 485–491 (2006).
Bittencourt, V. C. B. et al. An α-glucan of Pseudallescheria boydii is involved in fungal phagocytosis and toll-like receptor activation. J. Biol. Chem. 281, 22614–22623 (2006).
Acknowledgements
We gratefully acknowledge to the Max-Planck Society and the European Research Council (ERC Advanced Grant AUTOHEPARIN) for generous financial support. We thank Dr C.-F. Liang for providing a building block for the Globo-H synthesis.
Author information
Authors and Affiliations
Contributions
H.S.H. and P.H.S. conceived and designed the experiments; H.S.H. and M.H. performed the experiments and P.H.S. supervised the project. H.S.H., M.H. and P.H.S. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
P.H.S. is a shareholder of GlycoUniverse, a company involved in automated carbohydrate synthesis. H.S.H. and M.H. declare no competing financial interest.
Supplementary information
Supplementary Information
Supplementary Figures 1-118, Supplementary Tables 1-10, Supplementary Methods and Supplementary References (PDF 31567 kb)
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Hahm, H., Hurevich, M. & Seeberger, P. Automated assembly of oligosaccharides containing multiple cis-glycosidic linkages. Nat Commun 7, 12482 (2016). https://doi.org/10.1038/ncomms12482
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/ncomms12482
This article is cited by
-
Unravelling the structure of glycosyl cations via cold-ion infrared spectroscopy
Nature Communications (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.