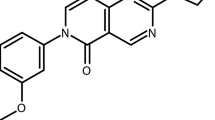
Abstract
Diseases caused by tropical parasites affect hundreds of millions of people worldwide but have been largely neglected for drug development because they affect poor people in poor regions of the world. Most of the current drugs used to treat these diseases are decades old and have many limitations, including the emergence of drug resistance. This review will summarize efforts to reinvigorate the drug development pipeline for these diseases, which is driven in large part by support from major philanthropies. The organisms responsible for these diseases have a fascinating biology, and many potential biochemical targets are now apparent. These neglected diseases present unique challenges to drug development that are being addressed by new consortia of scientists from academia and industry.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
McKerrow, J.H. Designing drugs for parasitic diseases of the developing world. PLoS Med. 2, e210 (2005).
Lipinski, C.A., Lombardo, F., Dominy, B.W. & Feeney, P.J. Experimenal and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 23, 3–25 (1997).
Wang, C.C. Validating targets for antiparasite chemotherapy. Parasitology 114 (suppl.), S31–S44 (1997).
Mackey, Z.B., O'Brien, T.C., Greenbaum, D.C., Blank, R.B. & McKerrow, J.H. A cathepsin B-like protease is required for host protein degradation in Trypanosoma brucei. J. Biol. Chem. 279, 48426–48433 (2004).
Bogyo, M., Verhelst, S., Bellingard-Dubouchaud, V., Toba, S. & Greenbaum, D. Selective targeting of lysosomal cysteine proteases with radiolabeled electrophilic substrate analogs. Chem. Biol. 7, 27–38 (2000).
Knight, Z.A. & Shokat, K.M. Features of selective kinase inhibitors. Chem. Biol. 12, 621–637 (2005).
Engel, J.C. et al. Cysteine protease inhibitors alter Golgi complex ultrastructure and function in Trypanosoma cruzi. J. Cell Sci. 111, 597–606 (1998).
McCann, P.P. & Pegg, A.E. Ornithine decarboxylase as an enzyme target for therapy. Pharmacol. Ther. 54, 195–215 (1992).
Werbovetz, K.A., Brendle, J.J., Boykin, D.W. & Stephens, C.E. Reversed amidines and methods of using them for treating, preventing, or inhibiting leishmaniasis. WO patent application 2002/036588 (2002).
Aschenbrenner, A. et al. Derivatives of diphenylurea, diphenyloxalic acid diamide and diphenylsulfuric acid diamide and their use as medicaments. WO patent application 2002070467 (2002).
Aschenbrenner, A. et al. Preparation of N-amidinophenyl-N'-sulfamoylphenylureas and related compounds for the treatment of protozoal diseases and as inhibitors of intracellular protein degradation pathways. US patent application 2003119876 (2003).
Werbovetz, K., Franzblau, S.G., Tidwell, R.R., Bakunova, S. & Bakunov, S. Cationic substituted benzofurans as antimicrobial agents. WO patent application 2005/055935 (2005).
Tidwell, R.R., Boykin, D., Brun, R., Stephens, C.E. & Kumar, A. Preparation of novel amidines for treating microbial infections like human African trypanosomiasis and falciparum malaria. WO patent application 2005/033065 (2005).
Boykin, D.W., Tidwell, R.R., Ismail, M.A. & Brun, R. Preparation of dicationic 2,5-diarylfuran aza-analogs as anti-protozoan agents. WO patent application 2004/050018 (2004).
Boykin, D.W. et al. Preparation of fused ring dicationic antiprotozoals and prodrugs thereof. WO patent application 2005/051296 (2005).
Brendle, J.J. et al. Antileishmanial activities of several classes of aromatic dications. Antimicrob. Agents Chemother. 46, 797–807 (2002).
Athri, P. et al. 3D QSAR on a library of heterocyclic diamidine derivatives with antiparasitic activity. Bioorg. Med. Chem. 14, 3144–3152 (2006).
Dardonville, C. et al. DNA binding affinity of bisguanidine and bis(2-aminoimidazoline) derivatives with in vivo antitrypanosomal activity. J. Med. Chem. 49, 3748–3752 (2006).
Boykin, D.W., Kumar, A., Hall, J.E., Bender, B.C. & Tidwell, R.R. Anti-pneumocystis activity of bisamidoximes and bis-O-alkylamidoximes prodrugs. Bioorg. Med. Chem. Lett. 6, 3017–3020 (1996).
Mayence, A. et al. Parallel solution-phase synthesis of conformationally restricted congeners of pentamidine and evaluation of their antiplasmodial activities. J. Med. Chem. 47, 2700–2705 (2004).
Rahmathullah, S.M. et al. Prodrugs for amidines: synthesis and anti-Pneumocystis carinii activity of aarbamates of 2,5-bis(4-amidinophenyl)furan. J. Med. Chem. 42, 3994–4000 (1999).
Zhou, L. et al. Enhanced permeability of the antimicrobial agent 2,5-bis(4-amidinophenyl)furan across Caco-2 cell monolayers via its methylamidoidme prodrug. Pharm. Res. 19, 1689–1695 (2002).
Sturk, L.M., Brock, J.L., Bagnell, C.R., Hall, J.E. & Tidwell, R.R. Distribution and quantitation of the anti-trypanosomal diamidine 2,5-bis(4-amidinophenyl)furan (DB75) and its N-methoxy prodrug DB289 in murine brain tissue. Acta Trop. 91, 131–143 (2004).
Berman, J.D. et al. Efficacy and safety of liposomal amphotericin B (AmBisome) for visceral leishmaniasis in endemic developing countries. Bull. World Health Organ. 76, 25–32 (1998).
Gibbs, W.J., Drew, R.H. & Perfect, J.R. Liposomal amphotericin B: clinical experience and perspectives. Expert Rev. Anti Infect. Ther. 3, 167–181 (2005).
Schweitzer, B.I., Dicker, A.P. & Bertino, J.R. Dihydrofolate reductase as a therapeutic target. FASEB J. 4, 2441–2452 (1990).
Ivanetich, K.M. & Santi, D.V. Thymidylate synthase-dihydrofolate reductase in protozoa. Exp. Parasitol. 70, 367–371 (1990).
Knighton, D.R. et al. Structure of and kinetic channelling in bifunctional dihydrofolate reductase-thymidylate synthase. Nat. Struct. Biol. 1, 186–194 (1994).
Zuccotto, F., Brun, R., Gonzalez Pacanowska, D., Ruiz Perez, L.M. & Gilbert, I.H. The structure-based design and synthesis of selective inhibitors of Trypanosoma cruzi dihydrofolate reductase. Bioorg. Med. Chem. Lett. 9, 1463–1468 (1999).
Zuccotto, F. et al. Novel inhibitors of Trypanosoma cruzi dihydrofolate reductase. Eur. J. Med. Chem. 36, 395–405 (2001).
Chowdhury, S.F. et al. Novel inhibitors of leishmanial dihydrofolate reductase. Bioorg. Med. Chem. Lett. 11, 977–980 (2001).
Gilbert, I.H. Inhibitors of dihydrofolate reductase in Leishmania and trypanosomes. Biochim. Biophys. Acta 1587, 249–257 (2002).
Nare, B., Hardy, L.W. & Beverley, S.M. The roles of pteridine reductase 1 and dihydrofolate reductase-thymidylate synthase in pteridine metabolism in the protozoan parasite Leishmania major. J. Biol. Chem. 272, 13883–13891 (1997).
Sirawaraporn, W. et al. Selective inhibition of Leishmania dihydrofolate reductase and Leishmania growth by 5-benzyl-2,4-diaminopyrimidines. Mol. Biochem. Parasitol. 31, 79–85 (1988).
Chowdhury, S.F. et al. Design, synthesis, and evaluation of inhibitors of trypanosomal and leishmanial dihydrofolate reductase. J. Med. Chem. 42, 4300–4312 (1999).
Pez, D. et al. 2,4-Diaminopyrimidines as inhibitors of leishmanial and trypanosomal dihydrofolate reductase. Bioorg. Med. Chem. 11, 4693–4711 (2003).
Hidalgo-Zarco, F. & Gonzalez-Pazanowska, D. Trypanosomal dUTPases as potential targets for drug design. Curr. Protein Pept. Sci. 2, 389–397 (2001).
Stout, C.D. Induced fit, drug design, and dUTPase. Structure 12, 2–3 (2004).
Whittingham, J.L. et al. dUTPase as a platform for antimalarial drug design: structural basis for the selectivity of a class of nucleoside inhibitors. Structure 13, 329–338 (2005).
Hofer, A., Steverding, D., Chabes, A., Brun, R. & Thelander, L. Trypanosoma brucei CTP synthetase: a target for the treatment of African sleeping sickness. Proc. Natl. Acad. Sci. USA 98, 6412–6416 (2001).
Sajid, M. & McKerrow, J.H. Review: cysteine proteases of parasitic organisms. Mol. Biochem. Parasitol. 120, 1–21 (2002).
Semenov, A., Olson, J.E. & Rosenthal, P.J. Antimalarial synergy of cysteine and aspartic protease inhibitors. Antimicrob. Agents Chemother. 42, 2254–2258 (1998).
Klemba, M. & Goldberg, D.E. Biological roles of proteases in parasitic protozoa. Annu. Rev. Biochem. 71, 275–305 (2002).
Chibale, K., Greenbaum, D.C. & McKerrow, J.H. Thiosemicarbazones and other compounds as antiparasitic compounds, their preparation, and methods of their use. WO patent application 2005/087211 (2005).
Du, X. et al. Synthesis and structure-activity relationship study of potent trypanocidal thio semicarbazone inhibitors of the trypanosomal cysteine protease cruzain. J. Med. Chem. 45, 2695–2707 (2002).
Selzer, P.M. et al. Cysteine protease inhibitors as chemotherapy: lessons from a parasite target. Proc. Natl. Acad. Sci. USA 96, 11015–11022 (1999).
Abdulla, M.-H., Lim, K.C., Sajid, M., McKerrow, J.H. & Caffrey, C.R. Schistosomiasis mansoni: novel chemotherapy using a cysteine protease inhibitor. PLoS Med. (in the press).
Burleigh, B.A. & Andrews, N.W. The mechanisms of Trypanosoma cruzi invasion of mammalian cells. Annu. Rev. Microbiol. 49, 175–200 (1995).
Salter, J.P. et al. Cercarial elastase is encoded by a functionally conserved gene family across multiple species of schistosomes. J. Biol. Chem. 277, 24618–24624 (2002).
Eggleson, K.K., Duffin, K.L. & Goldberg, D.E. Identification and characterization of falcilysin, a metallopeptidase involved in hemoglobin catabolism within the malaria parasite Plasmodium falciparum. J. Biol. Chem. 274, 32411–32417 (1999).
Madrid, P.B., Liou, A.P., DeRisi, J.L. & Guy, R.K. Incorporation of an intramolecular hydrogen-bonding motif in the side chain of 4-aminoquinolines enhances activity against drug-resistant P. falciparum. J. Med. Chem. 49, 4535–4543 (2006).
Krogstad, D.J. & De, D. Chloroquine analogs, their preparation, and methods of preventing and treating plasmodial disease. WO patent application 96/40138 (1996).
De, D., Krogstad, F.M., Byers, L.D. & Krogstad, D.J. Structure-activity relationships for antiplasmodial activity among 7-substituted 4-aminoquinolines. J. Med. Chem. 41, 4918–4926 (1998).
Ramanathan-Girish, S. et al. Pharmacokinetics of the antimalarial drug, AQ-13, in rats and cynomolgus macaques. Int. J. Toxicol. 23, 179–189 (2004).
Park, B.K., O'Neill, P.M., Ward, S.A. & Stocks, P.A. Preparation of anilino-quninolines as anti-malarial compounds. WO patent application 2002/072554 (2002).
Jain, R. et al. Ring-substituted 8-aminoquinoline analogs as antimalarial agents and process for their preparation. WO patent application 2004/085402 (2004).
Pratap, R. et al. Method for the treatment of malaria by the use of primaquine derivative N1-(3-ethylidinotetrahydrofuran-2-one)-N4-(6-methoxy-8-quinolinyl)-1,4-pentanediamine as gametocytocidal agent. US patent application 2003199697 (2003).
Sparatore, A. et al. Preparation of quinolizidinylalkylaminoquinolines as antimalarials. WO patent application 2005/037833 (2005).
Meunier, B., Robert, A., Dechy-Cabaret, O. & Benoit-Vical, F. Preparation of compounds which contain a 1,2,4-trioxane moiety linked to a quinoline moiety for pharmaceutical use as antimalarial agents. WO patent application 2001/077105 (2001).
Bathurst, I. & Hentschel, C. Medicines for Malaria Venture: sustaining antimalarial drug development. Trends Parasitol. 22, 301–307 (2006).
Haynes, R.K. et al. Highly antimalaria-active artemisinin derivatives: biological activity does not correlate with chemical reactivity. Angew. Chem. Int. Edn. Engl. 43, 1381–1385 (2004).
Cumming, J.N., Ploypradith, P. & Posner, G.H. Antimalarial activity of artemisinin (qinghaosu) and related trioxanes: mechanism(s) of action. Adv. Pharmacol. 37, 253–297 (1997).
Wu, Y. How might qinghaosu (artemisinin) and related compounds kill the intraerythrocytic malaria parasite? A chemist's view. Acc. Chem. Res. 35, 255–259 (2002).
Krishna, S., Uhlemann, A.-C. & Haynes, R.K. Artemisinins: mechanisms of action and potential for resistance. Drug Resist. Updat. 7, 233–244 (2004).
Eckstein-Ludwig, U. et al. Artemisinins target the SERCA of Plasmodium falciparum. Nature 424, 957–961 (2003).
O'Neill, P.M. et al. Enantiomeric 1,2,4-trioxanes display equivalent in vitro antimalarial activity versus Plasmodium falciparum malaria parasites: implications for the molecular mechanism of action of the artemisinins. ChemBioChem 6, 2048–2054 (2005).
O'Neill, P.M. & Posner, G.H. A medicinal chemistry perspective on artemisinin and related endoperoxides. J. Med. Chem. 47, 2945–2964 (2004).
Haynes, R.K. et al. Convenient access both to highly antimalaria-active 10-arylaminoartemisinins, and to 10-alkyl ethers including artemether, arteether, and artelinate. ChemBioChem 6, 659–667 (2005).
Haynes, R.K., Lam, W.-L., Chan, H.-W. & Tsang, H.-W. Preparation of artemisinin derivatives for treating malaria, neosporosis and coccidiosis. European patent application 974354 (2000).
O'Neill, P.M., Higson, A.P., Taylor, S. & Irving, E. Preparation of dihydroartemisinin derivatives as antimalarial and antitumor agents. WO patent application 2003/048167 (2003).
Posner, G.H. et al. Orally active, hydrolytically stable, semisynthetic, antimalarial trioxanes in the artemisinin family. J. Med. Chem. 42, 300–304 (1999).
O'Neill, P.M. et al. Novel, potent, semisynthetic antimalarial carba analogues of the first-generation 1,2,4-trioxane artemether. J. Med. Chem. 42, 5487–5493 (1999).
Begue, J.-P. et al. Preparation of novel artemisinin derivatives and their use for treating malaria. WO patent application 2003/035651 (2003).
Hindley, S. et al. Mechanism-based design of parasite-targeted artemisinin derivatives: synthesis and antimalarial activity of new diamine containing analogues. J. Med. Chem. 45, 1052–1063 (2002).
Posner, G.H. et al. Preparation of orally active, antimalarial, anticancer, artemisinin-derived trioxane dimers with high selectivity, stability and efficacy. WO patent application 2004/028476 (2004).
O'Neill, P.M., Higson, A.P., Taylor, S. & Irving, E. Preparation of dihydroartemisinin derivatives as antimalarial and anticancer agents. WO patent application 2003/048168 (2003).
Mahmoud, A.E. & Waseem, G. Preparation of anticancer and antiprotozoal dihydroartemisinene and dihydroartemisitene dimers with desirable chemical functionalities. WO patent application 2006/002105 (2006).
Haynes, R.K. & Lam, W.-L. Preparation of artemisinin derivatives as antiparasitic agents. European patent application 974593 (2000).
Avery, M.A. & Muraleedharan, K.M. Preparation of artemisinin-based peroxide compounds as broad spectrum anti-infective agents. WO patent application 2003/095444 (2003).
Haynes, R.K., Chan, H.-W., Lam, W.-L., Tsang, H.-W. & Cheung, M.-K. Synthesis and antiparasitic activity of artemisinin derivatives (endoperoxides). WO patent application 2000/004024 (2000).
Haynes, R.K. Preparation of antiparasitic artemisinin derivatives (sesquiterpene endoperoxides). WO patent application 2003/076446 (2003).
Haynes, R.K. et al. Artemisone - a highly active antimalarial drug of the artemisinin class. Angew. Chem. Int. Ed. 45, 2082–2088 (2006).
Medicines for Malaria Venture. Curing malaria together: annual report 2005. Medicines for Malaria Venture http://www.mmv.org/IMG/pdf/full_report.pdf (2005).
Posner, G.H. et al. Orally active antimalarial 3-substituted trioxanes: new synthetic methodology and biological evaluation. J. Med. Chem. 41, 940–951 (1998).
Singh, C., Malik, H. & Puri, S.K. Orally active 1,2,4-trioxanes: synthesis and antimalarial assessment of a new series of 9-functionalized 3-(1-arylvinyl)-1,2,5-trioxaspiro[5.5]undecanes against multi-drug-resistant Plasmodium yoelii nigeriensis in mice. J. Med. Chem. 49, 2794–2803 (2006).
Tripathi, R., Jefford, C.W. & Dutta, G.P. Blood schizontocidal activity of selected 1,2,4-trioxanes (fenozans) against the multidrug-resistant strain of Plasmodium yoelii nigeriensis (MDR) in vivo. Parasitology 133, 1–9 (2006).
Vennerstrom, J.L., Dong, Y., Chollet, J. & Matile, H. Preparation of spiro/dispiro-1,2,4-trioxolanes as antimalarial agents. US patent 6,486,199 (2002).
Vennerstrom, J.L. et al. Preparation of spiro- and dispiro-1,2,4-trioxolanes as antimalarial agents, schistosomicides, and anticancer agents. US patent application 2004039008 (2004).
Vennerstrom, J.L. et al. Identification of an antimalarial synthetic trioxolane drug development candidate. Nature 430, 900–904 (2004).
Dong, Y. et al. Spiro and dispiro-1,2,4-trioxolanes as antimalarial peroxides: charting a workable structure-activity relationship using simple prototypes. J. Med. Chem. 48, 4953–4961 (2005).
Vennerstrom, J.L. et al. Synthesis and antimalarial activity of sixteen dispiro-1,2,4, 5-tetraoxanes: alkyl-substituted 7,8,15,16-tetraoxadispiro[5.2.5. 2]hexadecanes. J. Med. Chem. 43, 2753–2758 (2000).
Kim, H.S. et al. Synthesis and antimalarial activity of novel medium-sized 1,2,4,5-tetraoxacycloalkanes. J. Med. Chem. 44, 2357–2361 (2001).
Posner, G.H. et al. Antimalarial cyclic peroxy ketals. J. Med. Chem. 41, 2164–2167 (1998).
Hofheinz, W. et al. Ro 42–1611 (arteflene), a new effective antimalarial: chemical structure and biological activity. Trop. Med. Parasitol. 45, 261–265 (1994).
Bachi, M.D. et al. A short synthesis and biological evaluation of potent and nontoxic antimalarial bridged bicyclic β-sulfonyl-endoperoxides. J. Med. Chem. 46, 2516–2533 (2003).
O'Neill, P.M. et al. Design and synthesis of endoperoxide antimalarial prodrug models. Angew. Chem. Int. Ed. 43, 4193–4197 (2004).
Posner, G.H. et al. Orally active antimalarial 3-substituted trioxanes: new synthetic methodology and biological evaluation. J. Med. Chem. 41, 940–951 (1998).
Posner, G.H., Parker, M.H., Krasavin, M. & Shapiro, T.A. Synthesis and activity of water-soluble trioxanes as potent and safe antimalarial agents. WO patent application 2000/059501 (2000).
Singh, C., Tiwari, P. & Puri, S.K. Novel substituted 1,2,4-trioxanes useful as antimalarial agents and a process for the preparation thereof. WO patent application 2003082852 (2003).
O'Neill, P.M., Amewu, R., Mukhtar, A. & Ward, S.A. 1,2,4-Trioxanes and 1,2,4-trioxepanes useful as antimalarial and anticancer agents, and their pharmaceutical compositions, use, and preparation via the thiol-olefin co-oxygenation (TOCO) reaction. US patent application 2005256184 (2005).
Szpilman, A.M., Korshin, E.E., Rozenberg, H. & Bachi, M.D. Total syntheses of yingzhaosu A and of its C(14)-epimer including the first evaluation of their antimalarial and cytotoxic activities. J. Org. Chem. 70, 3618–3632 (2005).
Vennerstrom, J.L. et al. Spiro and dispiro 1,2,4-trioxolane antimalarials, and their preparation, pharmaceutical compositions, and use in the treatment of malaria, cancer, and schistosomiasis. US patent application 2005256185 (2005).
Gelb, M.H. et al. Therapeutic intervention based on protein prenylation and associated modifications. Nat. Chem. Biol. 2, 518–528 (2006).
Yokoyama, K., Goodwin, G.W., Ghomashchi, F., Glomset, J. & Gelb, M.H. Protein prenyltransferases. Biochem. Soc. Trans. 20, 489–494 (1992).
Leonard, D.M. Ras farnesyltransferase: a new therapeutic target. J. Med. Chem. 40, 2971–2990 (1997).
Eastman, R.T., Buckner, F.S., Yokoyama, K., Gelb, M.H. & Van Voorhis, W.C. Fighting parasitic disease by blocking protein farnesylation. J. Lipid Res. 47, 233–240 (2006).
Gelb, M.H. et al. Protein farnesyl and N-myristoyl transferases: piggy-back medicinal chemistry targets for the development of antitrypanosomatid and antimalarial therapeutics. Mol. Biochem. Parasitol. 126, 155–163 (2003).
Nallan, L. et al. Protein farnesyltransferase inhibitors exhibit potent antimalarial activity. J. Med. Chem. 48, 3704–3713 (2005).
Glenn, M.P. et al. Structurally simple, potent, Plasmodium selective farnesyltransferase inhibitors that arrest the growth of malaria parasites. J. Med. Chem. 49, 5710–5727 (2006).
Opperdoes, F.R. & Borst, P. Localization of nine glycolytic enzymes in a microbody-like organelle in Trypanosoma brucei: the glycosome. FEBS Lett. 80, 360–364 (1977).
Clayton, C.E. & Michels, P. Metabolic compartmentation in African trypanosomes. Parasitol. Today 12, 465–471 (1996).
Opperdoes, F.R. & Michels, P.A. Enzymes of carbohydrate metabolism as potential drug targets. Int. J. Parasitol. 31, 482–490 (2001).
Verlinde, C.L.M.J. et al. Glycolysis as a target for the design of new anti-trypanosome drugs. Drug Resist. Updat. 4, 50–65 (2001).
Maertens, J.A. History of the development of azole derivatives. Clin. Microbiol. Infect. 10 (suppl.), 1–10 (2004).
Kale, P. & Johnson, L.B. Second-generation azole antifungal agents. Drugs Today (Barc) 41, 91–105 (2005).
Apt, W. et al. Treatment of chronic Chagas' disease with itraconazole and allopurinol. Am. J. Trop. Med. Hyg. 59, 133–138 (1998).
McCabe, R. Failure of ketoconazole to cure chronic murine Chagas' disease. J. Infect. Dis. 158, 1408–1409 (1988).
Brener, Z. et al. An experimental and clinical assay with ketoconazole in the treatment of Chagas disease. Mem. Inst. Oswaldo Cruz 88, 149–153 (1993).
Guedes, P.M. et al. Activity of the new triazole derivative albaconazole against Trypanosoma (Schizotrypanum) cruzi in dog hosts. Antimicrob. Agents Chemother. 48, 4286–4292 (2004).
Buckner, F.S., Wilson, A.J., White, T.C. & Van Voorhis, W.C. Induction of resistance to azole drugs in Trypanosoma cruzi. Antimicrob. Agents Chemother. 42, 3245–3250 (1998).
Hankins, E.G., Gillespie, J.R., Aikenhead, K. & Buckner, F.S. Upregulation of sterol C14-demethylase expression in Trypanosoma cruzi treated with sterol biosynthesis inhibitors. Mol. Biochem. Parasitol. 144, 68–75 (2005).
Urbina, J.A. et al. Antiproliferative effects and mechanism of action of SCH 56592 against Trypanosoma (Schizotrypanum) cruzi: in vitro and in vivo studies. Antimicrob. Agents Chemother. 42, 1771–1777 (1998).
Molina, J. et al. Activities of the triazole derivative SCH 56592 (posaconazole) against drug-resistant strains of the protozoan parasite Trypanosoma (Schizotrypanum) cruzi in immunocompetent and immunosuppressed murine hosts. Antimicrob. Agents Chemother. 44, 150–155 (2000).
Naula, C., Parsons, M. & Mottram, J.C. Protein kinases as drug targets in trypanosomes and Leishmania. Biochim. Biophys. Acta 1754, 151–159 (2005).
Bonn, D. New ways with old bones. Osteoporosis researchers look for drugs to replace hormone replacement therapy. Lancet 363, 786–787 (2004).
Cichewicz, R.H., Lim, K.C., McKerrow, J.H. & Nair, M.G. Kwanzoquinones A-G and other constituents of Hemerocallis fulva 'Kwanzo' roots and their acivity against the human trematode parasite, Schistosoma mansoni. Tetrahedron 58, 8597–8606 (2002).
Kohn, A.B., Anderson, P.A., Roberts-Misterly, J.M. & Greenberg, R.M. Schistosome calcium channel beta subunits. Unusual modulatory effects and potential role in the action of the antischistosomal drug praziquantel. J. Biol. Chem. 276, 36873–36876 (2001).
Wasilewski, M.M., Lim, K.C., Phillips, J. & McKerrow, J.H. Cysteine protease inhibitors block schistosome hemoglobin degradation in vitro and decrease worm burden and egg production in vivo. Mol. Biochem. Parasitol. 81, 179–189 (1996).
Mackey, Z.B. et al. Discovery of trypanocidal compounds by whole cell HTS of Trypanosoma brucei. Chem. Biol. Drug Des. 67, 355–363 (2006).
St. George, S., Bishop, J.V., Titus, R.G. & Selitrennikoff, C.P. Novel compounds active against Leishmania major. Antimicrob. Agents Chemother. 50, 474–479 (2006).
Weisman, J.L. et al. Searching for new antimalarial therapeutics amongst known drugs. Chem. Biol. Drug Des. 67, 409–416 (2006).
Chong, C.R., Chen, X., Shi, L., Liu, J.O. & Sullivan, D.J. A clinical drug library screen identifies astemizole as an antimalarial agent. Nat. Chem. Biol. 2, 415–416 (2006).
Acknowledgements
The authors are supported by the Sandler Family Supporting Foundation, the Bernard Osher Foundation, the Drugs for Neglected Diseases Initiative, and US National Institute of Allergy and Infectious Disease Tropical Disease Research Units grant AI-35707.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Table 1
Additional reviews and resources recommended by the authors. (DOC 28 kb)
Rights and permissions
About this article
Cite this article
Renslo, A., McKerrow, J. Drug discovery and development for neglected parasitic diseases. Nat Chem Biol 2, 701–710 (2006). https://doi.org/10.1038/nchembio837
Issue Date:
DOI: https://doi.org/10.1038/nchembio837
This article is cited by
-
Evaluation of the hydroalcoholic extract of Clarisia racemosa as an antiparasitic agent: an in vitro approach
3 Biotech (2023)
-
Cutaneous Leishmaniasis: A 2022 Updated Narrative Review into Diagnosis and Management Developments
American Journal of Clinical Dermatology (2022)
-
The Effect of BTK Inhibitor Ibrutinib on Leishmania infantum Infection In Vitro
Acta Parasitologica (2022)
-
Comparative transcriptomics and host-specific parasite gene expression profiles inform on drivers of proliferative kidney disease
Scientific Reports (2021)
-
Prevalence and Risk Factors of Human Leishmaniasis in Ethiopia: A Systematic Review and Meta-Analysis
Infectious Diseases and Therapy (2021)