Abstract
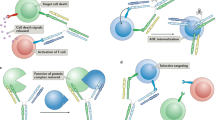
Chemically induced dimerizers (CIDs) have emerged as one of the most powerful tools for artificially regulating signaling pathways in cells; however, currently available CID systems lack the properties desired for use in regulating cellular therapies. Here, we report the development of human antibody-based chemically induced dimerizers (AbCIDs) from known small-molecule–protein complexes by selecting for synthetic antibodies that recognize the chemical epitope created by the bound small molecule. We demonstrate this concept by generating three antibodies that are highly selective for the BCL-xL–ABT-737 complex compared to BCL-xL alone. We show the potential of AbCIDs for application in regulating human cell therapies by using them to induce CRISPRa-mediated gene expression and to regulate CAR T-cell activation. We believe that the AbCIDs generated in this study will find application in regulating cell therapies and that the general method of AbCID development may lead to the creation of many new and orthogonal CIDs.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Spencer, D.M., Wandless, T.J., Schreiber, S.L. & Crabtree, G.R. Controlling signal transduction with synthetic ligands. Science 262, 1019–1024 (1993).
Fegan, A., White, B., Carlson, J.C. & Wagner, C.R. Chemically controlled protein assembly: techniques and applications. Chem. Rev. 110, 3315–3336 (2010).
DeRose, R., Miyamoto, T. & Inoue, T. Manipulating signaling at will: chemically-inducible dimerization (CID) techniques resolve problems in cell biology. Pflugers Arch. 465, 409–417 (2013).
Lienert, F., Lohmueller, J.J., Garg, A. & Silver, P.A. Synthetic biology in mammalian cells: next generation research tools and therapeutics. Nat. Rev. Mol. Cell Biol. 15, 95–107 (2014).
Shekhawat, S.S. & Ghosh, I. Split-protein systems: beyond binary protein-protein interactions. Curr. Opin. Chem. Biol. 15, 789–797 (2011).
Nguyen, D.P. et al. Ligand-binding domains of nuclear receptors facilitate tight control of split CRISPR activity. Nat. Commun. 7, 12009 (2016).
Straathof, K.C. et al. An inducible caspase 9 safety switch for T-cell therapy. Blood 105, 4247–4254 (2005).
Di Stasi, A. et al. Inducible apoptosis as a safety switch for adoptive cell therapy. N. Engl. J. Med. 365, 1673–1683 (2011).
DeFrancesco, L. CAR-T's forge ahead, despite Juno deaths. Nat. Biotechnol. 35, 6–7 (2017).
Rivera, V.M. et al. A humanized system for pharmacologic control of gene expression. Nat. Med. 2, 1028–1032 (1996).
Farrar, M.A., Alberol-Ila, J. & Perlmutter, R.M. Activation of the Raf-1 kinase cascade by coumermycin-induced dimerization. Nature 383, 178–181 (1996).
Miyamoto, T. et al. Rapid and orthogonal logic gating with a gibberellin-induced dimerization system. Nat. Chem. Biol. 8, 465–470 (2012).
Erhart, D. et al. Chemical development of intracellular protein heterodimerizers. Chem. Biol. 20, 549–557 (2013).
Kopytek, S.J., Standaert, R.F., Dyer, J.C. & Hu, J.C. Chemically induced dimerization of dihydrofolate reductase by a homobifunctional dimer of methotrexate. Chem. Biol. 7, 313–321 (2000).
Liang, F.S., Ho, W.Q. & Crabtree, G.R. Engineering the ABA plant stress pathway for regulation of induced proximity. Sci. Signal. 4, rs2 (2011).
Czlapinski, J.L. et al. Conditional glycosylation in eukaryotic cells using a biocompatible chemical inducer of dimerization. J. Am. Chem. Soc. 130, 13186–13187 (2008).
Schellekens, H. Factors influencing the immunogenicity of therapeutic proteins. Nephrol. Dial. Transplant. 20, vi3–vi9 (2005).
Lee, E.F. et al. Crystal structure of ABT-737 complexed with Bcl-xL: implications for selectivity of antagonists of the Bcl-2 family. Cell Death Differ. 14, 1711–1713 (2007).
Czabotar, P.E., Lessene, G., Strasser, A. & Adams, J.M. Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy. Nat. Rev. Mol. Cell Biol. 15, 49–63 (2014).
Besbes, S., Mirshahi, M., Pocard, M. & Billard, C. New dimension in therapeutic targeting of BCL-2 family proteins. Oncotarget 6, 12862–12871 (2015).
Oltersdorf, T. et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature 435, 677–681 (2005).
Van Duyne, G.D., Standaert, R.F., Karplus, P.A., Schreiber, S.L. & Clardy, J. Atomic structures of the human immunophilin FKBP-12 complexes with FK506 and rapamycin. J. Mol. Biol. 229, 105–124 (1993).
Hornsby, M. et al. A high through-put platform for recombinant antibodies to folded proteins. Mol. Cell. Proteomics 14, 2833–2847 (2015).
Shah, N.B. & Duncan, T.M. Bio-layer interferometry for measuring kinetics of protein-protein interactions and allosteric ligand effects. J. Vis. Exp. 2014, e51383 (2014).
Tse, C. et al. ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res. 68, 3421–3428 (2008).
Sattler, M. et al. Structure of Bcl-xL-Bak peptide complex: recognition between regulators of apoptosis. Science 275, 983–986 (1997).
Koerber, J.T., Hornsby, M.J. & Wells, J.A. An improved single-chain Fab platform for efficient display and recombinant expression. J. Mol. Biol. 427, 576–586 (2015).
Chavez, A. et al. Highly efficient Cas9-mediated transcriptional programming. Nat. Methods 12, 326–328 (2015).
Qi, L.S. et al. Repurposing CRISPR as an RNA-guided platform for sequence-specific control of gene expression. Cell 152, 1173–1183 (2013).
Gao, Y. et al. Complex transcriptional modulation with orthogonal and inducible dCas9 regulators. Nat. Methods 13, 1043–1049 (2016).
Fesnak, A.D., June, C.H. & Levine, B.L. Engineered T cells: the promise and challenges of cancer immunotherapy. Nat. Rev. Cancer 16, 566–581 (2016).
Wu, C.Y., Roybal, K.T., Puchner, E.M., Onuffer, J. & Lim, W.A. Remote control of therapeutic T cells through a small molecule-gated chimeric receptor. Science 350, aab4077 (2015).
Cao, Y. et al. Design of switchable chimeric antigen receptor T cells targeting breast cancer. Angew. Chem. Int. Ed. Engl. 55, 7520–7524 (2016).
Rodgers, D.T. et al. Switch-mediated activation and retargeting of CAR-T cells for B-cell malignancies. Proc. Natl. Acad. Sci. USA 113, E459–E468 (2016).
Ma, J.S. et al. Versatile strategy for controlling the specificity and activity of engineered T cells. Proc. Natl. Acad. Sci. USA 113, E450–E458 (2016).
June, C.H., Levine, B.L., Porter, D.L., Kalos, M.D. & Michael, M.C. Compositions and methods for treatment of cancer. US Patent 9,540,445 (2017).
Wei, P. et al. Bacterial virulence proteins as tools to rewire kinase pathways in yeast and immune cells. Nature 488, 384–388 (2012).
Ziegler, S.F., Ramsdell, F. & Alderson, M.R. The activation antigen CD69. Stem Cells 12, 456–465 (1994).
Smith-Garvin, J.E., Koretzky, G.A. & Jordan, M.S. T cell activation. Annu. Rev. Immunol. 27, 591–619 (2009).
Zhang, H. et al. Bcl-2 family proteins are essential for platelet survival. Cell Death Differ. 14, 943–951 (2007).
Goreshnik, I. & Maly, D.J. A small molecule-regulated guanine nucleotide exchange factor. J. Am. Chem. Soc. 132, 938–940 (2010).
Gao, J., Sidhu, S.S. & Wells, J.A. Two-state selection of conformation-specific antibodies. Proc. Natl. Acad. Sci. USA 106, 3071–3076 (2009).
Rizk, S.S. et al. Allosteric control of ligand-binding affinity using engineered conformation-specific effector proteins. Nat. Struct. Mol. Biol. 18, 437–442 (2011).
Staus, D.P. et al. Allosteric nanobodies reveal the dynamic range and diverse mechanisms of G-protein-coupled receptor activation. Nature 535, 448–452 (2016).
Thomsen, N.D., Koerber, J.T. & Wells, J.A. Structural snapshots reveal distinct mechanisms of procaspase-3 and -7 activation. Proc. Natl. Acad. Sci. USA 110, 8477–8482 (2013).
Barlow, A.D. et al. Rapamycin toxicity in MIN6 cells and rat and human islets is mediated by the inhibition of mTOR complex 2 (mTORC2). Diabetologia 55, 1355–1365 (2012).
Wang, B. et al. Rapamycin inhibiting Jurkat T cells viability through changing mRNA expression of serine/threonine protein phosphatase 2A. Transpl. Immunol. 26, 50–54 (2012).
Kelly, P.N., Grabow, S., Delbridge, A.R., Adams, J.M. & Strasser, A. Prophylactic treatment with the BH3 mimetic ABT-737 impedes Myc-driven lymphomagenesis in mice. Cell Death Differ. 20, 57–63 (2013).
Fiedler, M. & Skerra, A. in Handbook of Therapeutic Antibodies. Vol. 1. (eds. Dubel, S. & Reichert, J.M.) 435–474 (Wiley-VCH Verlag GmbH & Co. KgaA, 2014).
Kong, H.Y. & Byun, J. Nucleic acid aptamers: new methods for selection, stabilization, and application in biomedical science. Biomol. Ther. (Seoul) 21, 423–434 (2013).
Seiler, C.Y. et al. DNASU plasmid and PSI:Biology-Materials repositories: resources to accelerate biological research. Nucleic Acids Res. 42, D1253–D1260 (2014).
Rajan, S. et al. Structural transition in Bcl-xL and its potential association with mitochondrial calcium ion transport. Sci. Rep. 5, 10609 (2015).
ORFeome Collaboration. The ORFeome Collaboration: a genome-scale human ORF-clone resource. Nat. Methods 13, 191–192 (2016).
Gilbert, L.A. et al. CRISPR-mediated modular RNA-guided regulation of transcription in eukaryotes. Cell 154, 442–451 (2013).
Acknowledgements
We thank S. Sidhu (University of Toronto) for providing the phage-displayed Fab library. We thank A. Weiss (UCSF) and T. Kadlecek (UCSF) for kindly providing the NFAT-dependent GFP reporter Jurkat cell line. Funding was provided by R01 grants from the NIH (CA191018 and GM097316) and a P41 grant from the NCI (P41 CA196276). Z.B.H. was supported by a postdoctoral fellowship from the Helen Hay Whitney Foundation and HHMI, as well as a Pathway to Independence Award from the NIH-NCI (K99CA203002). A.J.M. was supported by a predoctoral fellowship from the NSF GRFP. D.P.N. is the Connie and Bob Lurie Fellow of the Damon Runyon Cancer Research Foundation (DRG-2204-14).
Author information
Authors and Affiliations
Contributions
Z.B.H. and A.J.M. performed all experiments except those explicitly stated. D.P.N. designed experiments, prepared constructs, prepared cell lines, and performed experiments related to small-molecule control of CRISPRa-mediated gene expression. Z.B.H., A.J.M., and J.A.W. designed the research and analyzed the data. Z.B.H., A.J.M., and J.A.W. wrote the paper. All authors edited the paper.
Corresponding author
Ethics declarations
Competing interests
Z.B.H., A.J.M., J.A.W., and the University of California, San Francisco have filed a patent application related to the technology described in this manuscript. The value of this patent application may be affected by publication of this manuscript.
Supplementary information
Supplementary Text and Figures
Supplementary Results, Supplementary Tables 1–3, Supplementary Figures 1–15 (PDF 5687 kb)
Rights and permissions
About this article
Cite this article
Hill, Z., Martinko, A., Nguyen, D. et al. Human antibody-based chemically induced dimerizers for cell therapeutic applications. Nat Chem Biol 14, 112–117 (2018). https://doi.org/10.1038/nchembio.2529
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.2529
This article is cited by
-
In vivo human T cell engineering with enveloped delivery vehicles
Nature Biotechnology (2024)
-
Small molecule-nanobody conjugate induced proximity controls intracellular processes and modulates endogenous unligandable targets
Nature Communications (2023)
-
Chemically inducible split protein regulators for mammalian cells
Nature Chemical Biology (2023)
-
A simeprevir-inducible molecular switch for the control of cell and gene therapies
Nature Communications (2023)
-
The lipid components of high-density lipoproteins (HDL) are essential for the binding and transportation of antimicrobial peptides in human serum
Scientific Reports (2022)