Abstract
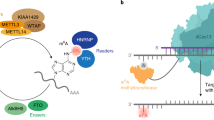
N6-methyladenosine (m6A) is the most prevalent and reversible internal modification in mammalian messenger and noncoding RNAs. We report here that human methyltransferase-like 14 (METTL14) catalyzes m6A RNA methylation. Together with METTL3, the only previously known m6A methyltransferase, these two proteins form a stable heterodimer core complex of METTL3–METTL14 that functions in cellular m6A deposition on mammalian nuclear RNAs. WTAP, a mammalian splicing factor, can interact with this complex and affect this methylation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Accession codes
References
Bokar, J.A. in Fine-Tuning of RNA Functions by Modification and Editing Vol. 12 (ed. Grosjean, H.), 141–177 (Springer-Verlag, Berlin Heidelberg, 2005).
Jia, G., Fu, Y. & He, C. Trends Genet. 29, 108–115 (2013).
Motorin, Y. & Helm, M. Wiley Interdiscip. Rev. RNA 2, 611–631 (2011).
Beemon, K. & Keith, J.J. Mol. Biol. 113, 165–179 (1977).
Jia, G. et al. Nat. Chem. Biol. 7, 885–887 (2011).
Zheng, G. et al. Mol. Cell 49, 18–29 (2013).
Dominissini, D. et al. Nature 485, 201–206 (2012).
Meyer, K.D. et al. Cell 149, 1635–1646 (2012).
Bokar, J.A., Shambaugh, M.E., Polayes, D., Matera, A.G. & Rottman, F.M. RNA 3, 1233–1247 (1997).
Clancy, M.J., Shambaugh, M.E., Timpte, C.S. & Bokar, J.A. Nucleic Acids Res. 30, 4509–4518 (2002).
Hongay, C.F. & Orr-Weaver, T.L. Proc. Natl. Acad. Sci. USA 108, 14855–14860 (2011).
Zhong, S. et al. Plant Cell 20, 1278–1288 (2008).
Bujnicki, J.M., Feder, M., Radlinska, M. & Blumenthal, R.M.J. J. Mol. Evol. 55, 431–444 (2002).
Horiuchi, K. et al. Proc. Natl. Acad. Sci. USA 103, 17278–17283 (2006).
Agarwala, S.D., Blitzblau, H.G., Hochwagen, A. & Fink, G.R. PLoS Genet. 8, e1002732 (2012).
Bokar, J.A., Rath-Shambaugh, M.E., Ludwiczak, R., Narayan, P. & Rottman, F. J. Biol. Chem. 269, 17697–17704 (1994).
Hafner, M. et al. Cell 141, 129–141 (2010).
Wang, X. et al. Nature doi:10.1038/nature12730 (27 November 2013).
Fustin, J.-M. et al. Cell 155, 793–806 (2013).
Yu, M. et al. Nat. Protoc. 7, 2159–2170 (2012).
Kane, S.E. & Beemon, K. Mol. Cell. Biol. 5, 2298–2306 (1985).
Bolte, S. & Cordelières, F.P. J. Microsc. 224, 213–232 (2006).
Hafner, M. et al. J. Vis. Exp. 41, e2034 (2010).
Dominissini, D. et al. Nat. Protoc. 8, 176–189 (2013).
Ascano, M. et al. Nature 492, 382–386 (2012).
Zhang, Y. et al. Genome Biol. 9, R137 (2008).
Acknowledgements
This study was supported by US National Institutes of Health (GM071440 and GM088599). We thank P. Faber and L. Dore for helping with high-throughput sequencing experiments and S.F. Reichard for editing the manuscript.
Author information
Authors and Affiliations
Contributions
C.H. conceived the project. J.L. and Y.Y. designed and performed most experiments. D.H. and Z.L. performed high-throughput sequencing data analyses. X.W. and Y.F. helped perform the PAR-CLIP experiment, biochemistry assay and data analysis. L.Z. and M.Y. assisted in expressing recombinant proteins in insect cells. G.J. and W.C. participated in subcloning. X.D. participated in nuclear extract separation. Q.D. synthesized the d3-m6A standard for LC/MS/MS analysis. J.L., Y.Y. and C.H. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Results, Supplementary Figures 1–15, Supplementary Tables 1–12 and Supplementary Notes 1–4. (PDF 3479 kb)
Supplementary Data Set 1
Summary of METTL3 target genes based on 4SU-PAR-CLIP (XLSX 298 kb)
Supplementary Data Set 2
Summary of METTL14 target genes based on 4SU-PAR-CLIP (XLSX 202 kb)
Supplementary Data Set 3
Summary of WTAP target genes based on 4SU-PAR-CLIP (XLSX 312 kb)
Supplementary Data Set 4
Summary of m6A peaks that show statistically significant decrease in the m6A-IP/input ratio upon METTL3 knockdown (P < 0.05) (XLSX 137 kb)
Supplementary Data Set 5
Summary of m6A peaks that show statistically significant decrease in the m6A-IP/input ratio upon METTL14 knockdown (P < 0.05) (XLSX 45 kb)
Supplementary Data Set 6
Summary of m6A peaks that show statistically significant decrease in the m6A-IP/input ratio upon WTAP knockdown (P < 0.05) (XLSX 48 kb)
Rights and permissions
About this article
Cite this article
Liu, J., Yue, Y., Han, D. et al. A METTL3–METTL14 complex mediates mammalian nuclear RNA N6-adenosine methylation. Nat Chem Biol 10, 93–95 (2014). https://doi.org/10.1038/nchembio.1432
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.1432
This article is cited by
-
The epigenetic downregulation of LncGHRLOS mediated by RNA m6A methylase ZCCHC4 promotes colorectal cancer tumorigenesis
Journal of Experimental & Clinical Cancer Research (2024)
-
A bibliometric analysis of m6A methylation in viral infection from 2000 to 2022
Virology Journal (2024)
-
N6-methyladenosine modification positively regulate Japanese encephalitis virus replication
Virology Journal (2024)
-
IGF2BP3 enhances lipid metabolism in cervical cancer by upregulating the expression of SCD
Cell Death & Disease (2024)
-
New horizons for the role of RNA N6-methyladenosine modification in hepatocellular carcinoma
Acta Pharmacologica Sinica (2024)