Abstract
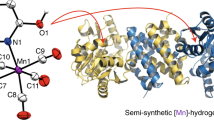
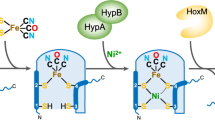
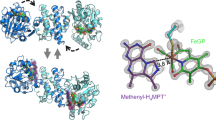
Hydrogenases catalyze the formation of hydrogen. The cofactor ('H-cluster') of [FeFe]-hydrogenases consists of a [4Fe-4S] cluster bridged to a unique [2Fe] subcluster whose biosynthesis in vivo requires hydrogenase-specific maturases. Here we show that a chemical mimic of the [2Fe] subcluster can reconstitute apo-hydrogenase to full activity, independent of helper proteins. The assembled H-cluster is virtually indistinguishable from the native cofactor. This procedure will be a powerful tool for developing new artificial H2-producing catalysts.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Frey, M. ChemBioChem 3, 153–160 (2002).
Peters, J.W. & Broderick, J.B. Annu. Rev. Biochem. 81, 429–450 (2012).
Peters, J.W., Lanzilotta, W.N., Lemon, B.J. & Seefeldt, L.C. Science 282, 1853–1858 (1998).
Posewitz, M.C. et al. J. Biol. Chem. 279, 25711–25720 (2004).
Mulder, D.W. et al. Nature 465, 248–251 (2010).
Czech, I., Silakov, A., Lubitz, W. & Happe, T. FEBS Lett. 584, 638–642 (2010).
Tard, C. & Pickett, C.J. Chem. Rev. 109, 2245–2274 (2009).
Tard, C. et al. Nature 433, 610–613 (2005).
Li, H. & Rauchfuss, T.B. J. Am. Chem. Soc. 124, 726–727 (2002).
Knörzer, P. et al. J. Biol. Chem. 287, 1489–1499 (2012).
Berggren, G. et al. Nature 499, 66–69 (2013).
Meyer, J. Cell Mol. Life Sci. 64, 1063–1084 (2007).
Nicolet, Y., Piras, C., Legrand, P., Hatchikian, C.E. & Fontecilla-Camps, J.C. Structure 7, 13–23 (1999).
Atta, M. & Meyer, J. Biochim. Biophys. Acta 1476, 368–371 (2000).
van Dijk, C. & Veeger, C. Eur. J. Biochem. 114, 209–219 (1981).
Winkler, M., Kuhlgert, S., Hippler, M. & Happe, T. J. Biol. Chem. 284, 36620–36627 (2009).
Noth, J., Krawietz, D., Hemschemeier, A. & Happe, T. J. Biol. Chem. 288, 4368–4377 (2013).
Lubitz, W., Reijerse, E. & van Gastel, M. Chem. Rev. 107, 4331–4365 (2007).
Kamp, C. et al. Biochim. Biophys. Acta 1777, 410–416 (2008).
Silakov, A., Kamp, C., Reijerse, E., Happe, T. & Lubitz, W. Biochemistry 48, 7780–7786 (2009).
Adamska, A. et al. Angew. Chem. Int. Ed. Engl. 51, 11458–11462 (2012).
Shepard, E.M. et al. J. Am. Chem. Soc. 132, 9247–9249 (2010).
Mulder, D.W. et al. Structure 19, 1038–1052 (2011).
Razavet, M. et al. Chem. Commun. (Camb.) 7, 700–701 (2002).
Winkler, M., Esselborn, J. & Happe, T. Biochim. Biophys. Acta 1827, 974–985 (2013).
Akhtar, M.K. & Jones, P.R. Appl. Microbiol. Biotechnol. 78, 853–862 (2008).
Kuchenreuther, J.M. et al. PLoS ONE 5, e15491 (2010).
von Abendroth, G. et al. Int. J. Hydrogen Energy 33, 6076–6081 (2008).
Gulis, G., Narasimhulu, K.V., Fox, L.N. & Redding, K.E. Photosynth. Res. 96, 51–60 (2008).
Kuhlgert, S., Drepper, F., Fufezan, C., Sommer, F. & Hippler, M. Biochemistry 51, 7297–7303 (2012).
Schmidt, M., Contakes, S.M. & Rauchfuss, T.B. J. Am. Chem. Soc. 121, 9736–9737 (1999).
Razavet, M. et al. Dalton Trans. 2003, 586–595 (2003).
Hemschemeier, A., Melis, A. & Happe, T. Photosynth. Res. 102, 523–540 (2009).
Zijlstra, W.G. & Buursma, A. Comp. Biochem. Physiol. B 118, 743–749 (1997).
Reijerse, E., Lendzian, F., Isaacson, R. & Lubitz, W. J. Magn. Reson. 214, 237–243 (2012).
Acknowledgements
This work was supported by the Bundesministerium für Bildung und Forschung Bio-H2 project (to T.H. and W.L.), the Max Planck Society, the French National Research Agency (NiFe–Cat ANR–10–BLAN–711 and Labex Program ARCANE 11–LABX–003 to T.S.,V.A. and M.F.) and the European Research Council under the European Union's Seventh Framework Programme (FP/2007-2013/ERC grant agreement no. 306398 to V.A.). G.B. gratefully acknowledges the Bengt Lundqvist Minnesfond, The Swedish Research Council for Environment, Agricultural Sciences and Spatial Planning (contract no. 213-2010-563) and the Swedish Royal Academy of Sciences. T.H. gratefully acknowledges support from the Deutsche Forschungsgemeinschaft (HA 255/2-1) and the Volkswagen foundation (LigH2t). J.E. is financed by the Studienstiftung des deutschen Volkes.
Author information
Authors and Affiliations
Contributions
C.L., J.E., J.N., A.H., M.F., W.L. and T.H. conceived and designed experiments. C.L., J.S., J.E., J.N. and A.A. performed the experiments. C.L., J.E., J.N., A.H., A.A. and T.H. analyzed the data. G.B., T.S., V.A. and M.F. provided the [2Fe]MIM and [2Fe]pdt complexes. A.A., E.R. and W.L. performed and analyzed the EPR and FTIR experiments. All of the authors discussed the results. A.H., J.E. and T.H. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Results and Supplementary Figures 1–3. (PDF 792 kb)
Rights and permissions
About this article
Cite this article
Esselborn, J., Lambertz, C., Adamska-Venkatesh, A. et al. Spontaneous activation of [FeFe]-hydrogenases by an inorganic [2Fe] active site mimic. Nat Chem Biol 9, 607–609 (2013). https://doi.org/10.1038/nchembio.1311
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.1311
This article is cited by
-
Stepwise assembly of the active site of [NiFe]-hydrogenase
Nature Chemical Biology (2023)
-
A personal account on 25 years of scientific literature on [FeFe]-hydrogenase
JBIC Journal of Biological Inorganic Chemistry (2023)
-
Changing the tracks: screening for electron transfer proteins to support hydrogen production
JBIC Journal of Biological Inorganic Chemistry (2022)
-
Azadithiolate-bridged [FeFe]-hydrogenase mimics with bridgehead N-derivation: structural and electrochemical investigations
Transition Metal Chemistry (2022)
-
Stability of the H-cluster under whole-cell conditions—formation of an Htrans-like state and its reactivity towards oxygen
JBIC Journal of Biological Inorganic Chemistry (2022)