Abstract
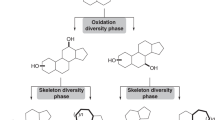
Nature has exploited medium-sized 8- to 11-membered rings in a variety of natural products to address diverse and challenging biological targets. However, owing to the limitations of conventional cyclization-based approaches to medium-ring synthesis, these structures remain severely underrepresented in current probe and drug discovery efforts. To address this problem, we have established an alternative, biomimetic ring expansion approach to the diversity-oriented synthesis of medium-ring libraries. Oxidative dearomatization of bicyclic phenols affords polycyclic cyclohexadienones that undergo efficient ring expansion to form benzannulated medium-ring scaffolds found in natural products. The ring expansion reaction can be induced using three complementary reagents that avoid competing dienone-phenol rearrangements and is driven by rearomatization of a phenol ring adjacent to the scissile bond. Cheminformatic analysis of the resulting first-generation library confirms that these molecules occupy chemical space overlapping with medium-ring natural products and distinct from that of synthetic drugs and drug-like libraries.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Macías, F.A., Varela, R.M., Torres, A., Molinillo, J.M.G. & Fronczek, F.R. Allelopathic studies on cultivar species. 2. Novel sesquiterpene from bioactive fractions of cultivar sunflowers. Tetrahedr. Lett. 34, 1999–2002 (1993).
Shimokawa, T., Kinjo, J., Yamahara, J., Yamasaki, M. & Nohara, T. Two novel aromatic compounds from Caesalpinia sappan. Chem. Pharm. Bull. (Tokyo) 33, 3545–3547 (1985).
Singh, S.B. et al. Aspercyclide A–C, three novel fungal metabolites from Aspergillus sp. as inhibitors of high-affinity IgE receptor. Tetrahedr. Lett. 45, 7605–7608 (2004).
Shirataki, Y., Tagaya, Y., Yokoe, I. & Komatsu, M. Studies on the constituents of Sophora species. Part 21. Sophoraside A, a new aromatic glycoside from the roots of Sophora japonica. Chem. Pharm. Bull. (Tokyo) 35, 1637–1640 (1987).
Fu, X. et al. Flavanone and chalcone derivatives from Cryptocarya kurzii. J. Nat. Prod. 56, 1153–1163 (1993).
Quideau, S. & Feldman, K.S. Ellagitannin chemistry. Chem. Rev. 96, 475–504 (1996).
Kupchan, S.M. et al. Tumor inhibitors. LXXX. Steganacin and steganangin, novel antileukemic lignan lactones from Steganotaenia araliacea. J. Am. Chem. Soc. 95, 1335–1336 (1973).
Abraham, D.J., Rosenstein, R.D., Lyon, R.L. & Fong, H.H.S. The structure elucidation of rhazinilam, a new class of alkaloids from the Apocynaceae, by X-ray analysis. Tetrahedr. Lett. 13, 909–912 (1972).
Majhi, T.P., Achari, B. & Chattopadhyay, P. Advances in the synthesis and biological perspectives of benzannulated medium ring heterocycles. Heterocycles 71, 1011–1052 (2007).
Khan, A.R. et al. Lowering the entropic barrier for binding conformationally flexible inhibitors to enzymes. Biochemistry 37, 16839–16845 (1998).
Benfield, A.P. et al. Ligand preorganization may be accompanied by entropic penalties in protein-ligand interactions. Angew. Chem. Int. Ed. Engl. 45, 6830–6835 (2006).
Udugamasooriya, D.G. & Spaller, M.R. Conformational constraint in protein ligand design and the inconsistency of binding entropy. Biopolymers 89, 653–667 (2008).
Veber, D.F. et al. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 45, 2615–2623 (2002).
Rezai, T., Yu, B., Millhauser, G.L., Jacobson, M.P. & Lokey, R.S. Testing the conformational hypothesis of passive membrane permeability using synthetic cyclic peptide diastereomers. J. Am. Chem. Soc. 128, 2510–2511 (2006).
Kwon, Y.-U. & Kodadek, T. Quantitative comparison of the relative cell permeability of cyclic and linear peptides. Chem. Biol. 14, 671–677 (2007).
McGrath, N.A., Brichacek, M. & Njardarson, J.T. A graphical journey of innovative organic architectures that have improved our lives. J. Chem. Educ. 87, 1348–1349 (2010).
Illuminati, G. & Mandolini, L. Ring closure reactions of bifunctional chain molecules. Acc. Chem. Res. 14, 95–102 (1981).
Bauer, R.A., Wurst, J.M. & Tan, D.S. Expanding the range of ′druggable′ targets with natural product–based libraries: an academic perspective. Curr. Opin. Chem. Biol. 14, 308–314 (2010).
Fürstner, A. & Mueller, C. Total synthesis of aspercyclide C. Chem. Commun. (Camb.) 5583–5585 (2005).10.1039/B512877C
Pospíšil, J., Mueller, C. & Fürstner, A. Total synthesis of the aspercyclides. Chemistry 15, 5956–5968 (2009).
Stefinovic, M. & Snieckus, V. Connecting directed ortho metalation and olefin metathesis strategies. Benzene-fused multiring-sized oxygen heterocycles. First syntheses of radulanin A and helianane. J. Org. Chem. 63, 2808–2809 (1998).
Kishuku, H., Shindo, M. & Shishido, K. Enantioselective total synthesis of (–)-heliannuol A. Chem. Commun. (Camb.) 350–351 (2003).10.1039/B211227B
Biswas, B., Sen, P.K. & Venkateswaran, R.V. Bargellini condensation of coumarins. Expeditious route to [o-(carboxyvinyl)phenoxy]isobutyric acids and application to the synthesis of sesquiterpenes helianane, heliannuol A and heliannuol C. Tetrahedron 63, 12026–12036 (2007).
Macías, F.A., Chinchilla, D., Molinillo, J.M.G., Fronczek, F.R. & Shishido, K. A stereoselective route towards heliannuol A. Tetrahedron 64, 5502–5508 (2008).
Sabui, S., Ghosh, S., Sarkar, D. & Venkateswaran, R.V. Expeditious synthesis of helianane and C-10 halogenated heliananes employing ring-closing metathesis. Tetrahedr. Lett. 50, 4683–4684 (2009).
Deb, I., John, S. & Namboothiri, I.N.N. Synthesis of benzo-fused medium ring cyclic ethers via a Michael addition–ring closing metathesis strategy involving nitroaliphatic compounds. Tetrahedron 63, 11991–11997 (2007).
Souers, A.J., Virgilio, A.A., Rosenquist, A., Fenuik, W. & Ellman, J.A. Identification of a potent heterocyclic ligand to somatostatin receptor subtype 5 by the synthesis and screening of β-turn mimetic libraries. J. Am. Chem. Soc. 121, 1817–1825 (1999).
Spring, D.R., Krishnan, S., Blackwell, H.E. & Schreiber, S.L. Diversity-oriented synthesis of biaryl-containing medium rings using a one bead/one stock solution platform. J. Am. Chem. Soc. 124, 1354–1363 (2002).
Khadem, S. et al. A solution- and solid-phase approach to tetrahydroquinoline-derived polycyclics having a 10-membered ring. J. Comb. Chem. 6, 724–734 (2004).
Brown, N. et al. Design and synthesis of medium-ring lactam libraries inspired by octalactin A. A convergent-divergent approach. J. Comb. Chem. 10, 628–631 (2008).
Marcaurelle, L.A. et al. An aldol-based build/couple/pair strategy for the synthesis of medium- and large-sized rings: discovery of macrocyclic histone deacetylase inhibitors. J. Am. Chem. Soc. 132, 16962–16976 (2010).
Roxburgh, C.J. Syntheses of medium sized rings by ring expansion reactions. Tetrahedron 49, 10749–10784 (1993).
Hesse, M. Ring Enlargement in Organic Chemistry (VCH, Weinheim, 1991).
Barton, D.H.R. Some studies in the biogenesis of plant products. Pure Appl. Chem. 9, 35–48 (1964).
Battersby, A.R., Bhatnagar, A.K., Hackett, P., Thornber, C.W. & Staunton, J. Synthesis of protostephanine by a route related to the biosynthetic pathway. Chem. Commun. 1214–1215 (1968).10.1039/C19680001214
Bhakuni, D.S. & Jain, S. The biosynthesis of laurifinine. Tetrahedron 37, 3171–3174 (1981).
Theuns, H.G. et al. Neodihydrothebaine and bractazonine, two dibenz[d,f]azonine alkaloids of Papaver bracteatum. Phytochemistry 23, 1157–1166 (1984).
Marino, J.P. & Samanen, J.M. Biogenetic-type approach to homoerythrina alkaloids. J. Org. Chem. 41, 179–180 (1976).
Kupchan, S.M., Kim, C.-K. & Lynn, J.T. Biomimetic synthesis of a key Erythrina alkaloid precursor. J. Chem. Soc. Chem. Commun. 86 (1976).10.1039/C3976000086A
Kupchan, S.M., Dhingra, O.P. & Kim, C.-K. New biogenetic-type approach to Cephalotaxus alkaloids. J. Chem. Soc. Chem. Commun. 847–848 (1977).10.1039/C39770000847
Kopp, F., Stratton, C.F., Akella, L.B. & Tan, D.S. A diversity-oriented synthesis approach to macrocycles via oxidative ring expansion. Nat. Chem. Biol. 8, 358–365 (2012).
Miller, B. Too many rearrangements of cyclohexadienones. Acc. Chem. Res. 8, 245–256 (1975).
Marino, J.P. & Samanen, J.M. Chemistry of prohomoerythrinadienone I. Tetrahedr. Lett. 14, 4553–4556 (1973).
Hosomi, A. Characteristics in the reactions of allylsilanes and their applications to versatile synthetic equivalents. Acc. Chem. Res. 21, 200–206 (1988).
Becker, H.D., Bremholt, T. & Adler, E. Oxidative formation and photochemical isomerization of spiro-epoxy-2,4-cyclohexadienones. Tetrahedr. Lett. 13, 4205–4208 (1972).
Morton, J.G.M., Kwon, L.D., Freeman, J.D. & Njardarson, J.T. An Adler-Becker oxidation approach to vinigrol. Tetrahedr. Lett. 50, 1684–1686 (2009).
MacMillan, K.S. & Boger, D.L. Fundamental relationships between structure, reactivity, and biological activity for the duocarmycins and CC-1065. J. Med. Chem. 52, 5771–5780 (2009).
Roush, W.R., Gillis, H.R. & Essenfeld, A.P. Hydrofluoric acid catalyzed intramolecular Diels-Alder reactions. J. Org. Chem. 49, 4674–4682 (1984).
Harmata, M. & Rashatasakhon, P. Cycloaddition reactions of vinyl oxocarbenium ions. Tetrahedron 59, 2371–2395 (2003).
Moura-Letts, G., DiBlasi, C.M., Bauer, R.A. & Tan, D.S. Solid-phase synthesis and chemical space analysis of a 190-membered alkaloid/terpenoid-like library. Proc. Natl. Acad. Sci. USA 108, 6745–6750 (2011).
Acknowledgements
This work is dedicated to the memory of our colleague and mentor, David Y. Gin (1967–2011). We thank D. Boger for helpful discussions; C. Stratton for assistance with PCA calculations; J. Njar∂arson for providing drug structures; and G. Sukenick, H. Liu, H. Fang and S. Rusli for expert mass spectral analyses. Instant JChem was generously provided by ChemAxon. Financial support from the US National Institutes of Health (P41 GM076267 to D.S.T., P41 GM076267-03S1 to R.A.B. and T32 CA062948-Gudas to T.A.W.), W.H. Goodwin and A. Goodwin and the Commonwealth Foundation for Cancer Research, the Memorial Sloan-Kettering Cancer Center Experimental Therapeutics Center and the Tri-Institutional Stem Cell Initiative is gratefully acknowledged.
Author information
Authors and Affiliations
Contributions
R.A.B., T.A.W. and D.S.T. designed the experiments, analyzed the data and wrote the manuscript. R.A.B. and T.A.W. performed the synthesis and characterization. T.A.W. performed the molecular modeling studies. R.A.B. performed the PCA analysis.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Results, Supplementary Notes 1–3 (PDF 90779 kb)
Supplementary Data Set 1
PCA analysis (XLS 7676 kb)
Rights and permissions
About this article
Cite this article
Bauer, R., Wenderski, T. & Tan, D. Biomimetic diversity-oriented synthesis of benzannulated medium rings via ring expansion. Nat Chem Biol 9, 21–29 (2013). https://doi.org/10.1038/nchembio.1130
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.1130
This article is cited by
-
A general strategy for diversifying complex natural products to polycyclic scaffolds with medium-sized rings
Nature Communications (2019)
-
N-(Hetero)aryl-2-imidazolines: an emerging privileged motif for contemporary drug design
Chemistry of Heterocyclic Compounds (2017)
-
Synthesis of new pyrrolo[3,2-l]acridinones and pyrrolo[3,2-c][1,8]naphthyridinones by condensation of methoxybenzenes or phenols with isobutyric aldehyde and o-aminonitriles
Chemistry of Heterocyclic Compounds (2017)
-
Diversity-oriented synthetic strategy for developing a chemical modulator of protein–protein interaction
Nature Communications (2016)
-
Radical aryl migration enables diversity-oriented synthesis of structurally diverse medium/macro- or bridged-rings
Nature Communications (2016)