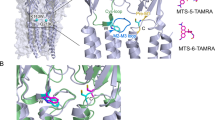
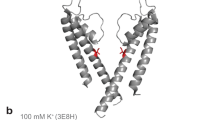
Abstract
In ion channels, 'rings' of ionized side chains that decorate the walls of the permeation pathway often lower the energetic barrier to ion conduction. Using single-channel electrophysiological recordings, we studied the poorly understood ring of four glutamates (and one glutamine) that dominates this catalytic effect in the muscle nicotinic acetylcholine receptor ('the intermediate ring of charge'). We show that all four wild-type glutamate side chains are deprotonated in the range of 6.0–9.0 pH, that only two of them contribute to the size of the single-channel current, that these side chains must be able to adopt alternate conformations that either allow or prevent their negative charges from increasing the rate of cation conduction and that the location of these glutamate side chains squarely at one of the ends of the transmembrane pore is critical for their largely unshifted pKa values and for the unanticipated impact of their conformational flexibility on cation permeation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Imoto, K. et al. Rings of negatively charged amino acids determine the acetylcholine receptor channel conductance. Nature 335, 645–648 (1988).
Galzi, J.L. et al. Mutations in the ion channel domain of a neuronal nicotinic receptor convert ion selectivity from cationic to anionic. Nature 359, 500–505 (1992).
Bertrand, D., Galzi, J.L., Devillers-Thiéry, A., Bertrand, S. & Changeux, J.P. Mutations at two distinct sites within the channel domain M2 alter calcium permeability of neuronal α7 nicotinic receptor. Proc. Natl. Acad. Sci. USA 90, 6971–6975 (1993).
Corringer, P.-J. Mutational analysis of the charge selectivity filter of the α7 nicotinic acetylcholine receptor. Neuron 22, 831–843 (1999).
Prod'hom, B., Pietrobon, D. & Hess, P. Direct measurement of proton transfer rates to a group controlling the dihydropyridine-sensitive Ca2+ channel. Nature 329, 243–246 (1987).
Root, M.J. & MacKinnon, R. Two identical noninteracting sites in an ion channel revealed by proton transfer. Science 265, 1852–1856 (1994).
Chen, X.-H., Bezprozvanny, I. & Tsien, R.W. Molecular basis of proton block of L-type Ca2+ channels. J. Gen. Physiol. 108, 363–374 (1996).
Hess, P., Lansman, J.B. & Tsien, R.W. Calcium channel selectivity for divalent and monovalent cations. Voltage and concentration dependence of single channel current in ventricular heart cells. J. Gen. Physiol. 88, 293–319 (1986).
Elenes, S., Decker, M., Cymes, G.D. & Grosman, C. Decremental response to high-frequency trains of acetylcholine pulses but unaltered fractional Ca2+ currents in a panel of 'slow-channel syndrome' nicotinic-receptor mutants. J. Gen. Physiol. 133, 151–169 (2009).
McDaniel, D.H. & Brown, H.C. Hydrogen bonding as a factor in the ionization of dicarboxylic acids. Science 118, 370–372 (1953).
Rajasekaran, E., Jayaram, B. & Honig, B. Electrostatic interactions in the aliphatic dicarboxylic acids: a computational route to the determination of pKa shifts. J. Am. Chem. Soc. 116, 8238–8240 (1994).
Flocco, M.M. & Mowbray, S.L. Strange bedfellows: interactions between acidic side chains in proteins. J. Mol. Biol. 254, 96–105 (1995).
Wohlfahrt, G. Analysis of pH-dependent elements in proteins: geometry and properties of pairs of hydrogen-bonded carboxylic acid side-chains. Proteins 58, 396–406 (2005).
Cymes, G.D., Ni, Y. & Grosman, C. Probing ion-channel pores one proton at a time. Nature 438, 975–980 (2005).
Cymes, G.D. & Grosman, C. Estimating the pKa values of basic and acidic side chains in ion channels using electrophysiological recordings: A robust approach to an elusive problem. Proteins 79, 3485–3493 (2011).
Bashford, D. & Gerwert, K. Electrostatic calculations of the pKa values of ionizable groups in bacteriorhodopsin. J. Mol. Biol. 224, 473–486 (1992).
Lancaster, C.R., Michel, H., Honig, B. & Gunner, M.R. Calculated coupling of electron and proton transfer in the photosynthetic reaction center of Rhodopseudomonas viridis. Biophys. J. 70, 2469–2492 (1996).
Qin, J., Clore, M. & Gronenborn, A. Ionization equilibria for side-chain carboxyl groups in oxidized and reduced human thioredoxin and in the complex with its target peptide from the transcription factor NFκB. Biochemistry 35, 7–13 (1996).
Lindman, S., Linse, S., Mulder, F.A. & André, I. Electrostatic contributions to residue-specific protonation equilibria and proton binding capacitance for a small protein. Biochemistry 45, 13993–14002 (2006).
Søndergaard, C.R., McIntosh, L.P., Pollastri, G. & Nielsen, J.E. Determination of electrostatic interaction energies and protonation state populations in enzyme active sites. J. Mol. Biol. 376, 269–287 (2008).
Bombarda, E. & Ullmann, G.M. pH-dependent pKa values in proteins—a theoretical analysis of protonation energies with practical consequences for enzymatic reactions. J. Phys. Chem. B 114, 1994–2003 (2010).
Bell, R.P. Kinetic isotope effects in proton-transfer reactions in The Proton in Chemistry 2nd edn. 250–296 (Cornell University Press, 1973).
Dahlgren, G. Jr. & Long, F.A. Relative hydrogen bonding of deuterium. I. Ionization constants of maleic and fumaric acids and of their monoethyl esters in H2O and D2O. J. Am. Chem. Soc. 82, 1303–1308 (1960).
Glasoe, P.K. & Eberson, L. Deuterium isotope effect in the ionization of substituted succinic acids in water and in deuterium oxide. J. Phys. Chem. 68, 1560–1562 (1964).
Glasoe, P.K. & Hutchison, J.R. Deuterium isotope effect in the ionization of substituted malonic acids in water and in deuterium oxide. J. Phys. Chem. 68, 1562–1563 (1964).
Salomaa, P., Schaleger, L.L. & Long, F.A. Solvent deuterium isotope effects on acid-base equilibria. J. Am. Chem. Soc. 86, 1–7 (1964).
Hilf, R.J. & Dutzler, R. X-ray structure of a prokaryotic pentameric ligand-gated ion channel. Nature 452, 375–379 (2008).
Bocquet, N. et al. X-ray structure of a pentameric ligand-gated ion channel in an apparently open conformation. Nature 457, 111–114 (2009).
Hilf, R.J. & Dutzler, R. Structure of a potentially open state of a proton-activated pentameric ligand-gated ion channel. Nature 457, 115–118 (2009).
Gutman, M., Tsfadia, Y., Masad, A. & Nachliel, E. Quantitation of physical-chemical properties of the aqueous phase inside the phoE ionic channel. Biochim. Biophys. Acta 1109, 141–148 (1992).
Fayer, M.D. & Levinger, N.E. Analysis of water in confined geometries and at interfaces. Annu. Rev. Anal. Chem. (Palo Alto Calif.) 3, 89–107 (2010).
Elenes, S., Ni, Y., Cymes, G.D. & Grosman, C. Desensitization contributes to the synaptic response of gain-of-function mutants of the muscle nicotinic receptor. J. Gen. Physiol. 128, 615–627 (2006).
Glasoe, P.K. & Long, F.A. Use of glass electrodes to measure acidities in deuterium oxide. J. Phys. Chem. 64, 188–190 (1960).
Qin, F. Restoration of single-channel currents using the segmental k-means method based on hidden Markov modeling. Biophys. J. 86, 1488–1501 (2004).
Qin, F., Auerbach, A. & Sachs, F. Estimating single-channel kinetic parameters from idealized patch-clamp data containing missed events. Biophys. J. 70, 264–280 (1996).
Ohno, K. et al. Congenital myasthenic syndrome caused by prolonged acetylcholine receptor channel openings due to a mutation in the M2 domain of the epsilon subunit. Proc. Natl. Acad. Sci. USA 92, 758–762 (1995).
Emsley, P., Lohkamp, B., Scott, W.G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 66, 486–501 (2010).
Humphrey, W., Dalke, A. & Schulten, K. VMD: visual molecular dynamics. J. Mol. Graph. 14, 33–38 (1996).
Lovell, S.C., Word, J.M., Richardson, J.S. & Richardson, D.C. The penultimate rotamer library. Proteins 40, 389–408 (2000).
Acknowledgements
We thank S. Sine (Mayo Clinic College of Medicine, Rochester, MN, USA) for wild-type muscle AChR cDNA, M. Slaughter (University at Buffalo School of Medicine, Buffalo, NY, USA) for wild-type α1 GlyR cDNA, and A. Holmstrom, M. Pasquini, J. Pizarek and M. Rigby for technical assistance. This work was supported by a grant from the US National Institutes of Health (R01-NS042169 to C.G.).
Author information
Authors and Affiliations
Contributions
G.D.C. and C.G. designed experiments, analyzed data and wrote the manuscript. G.D.C. performed experiments.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Methods and Supplementary Results (PDF 1590 kb)
Rights and permissions
About this article
Cite this article
Cymes, G., Grosman, C. The unanticipated complexity of the selectivity-filter glutamates of nicotinic receptors. Nat Chem Biol 8, 975–981 (2012). https://doi.org/10.1038/nchembio.1092
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.1092
This article is cited by
-
X-ray structure of the human α4β2 nicotinic receptor
Nature (2016)
-
X-ray structure of the mouse serotonin 5-HT3 receptor
Nature (2014)
-
Structural basis for ion permeation mechanism in pentameric ligand-gated ion channels
The EMBO Journal (2013)
-
Rotamers affect ion conductance
Nature Chemical Biology (2012)