Abstract
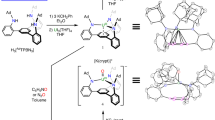
The oxo groups in the uranyl ion [UO2]2+—one of many oxo cations formed by metals from across the periodic table—are particularly inert, which explains the dominance of this ion in the laboratory and its persistence as an environmental contaminant. In contrast, transition metal oxo (M=O) compounds can be highly reactive and carry out difficult reactions such as the oxygenation of hydrocarbons. Here we show how the sequential addition of a lithium metal base to the uranyl ion constrained in a ‘Pacman’ environment results in lithium coordination to the U=O bonds and single-electron reduction. This reaction depends on the nature and stoichiometry of the lithium reagent and suggests that competing reduction and C–H bond activation reactions are occurring.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Denning, R. G. Electronic structure and bonding in actinyl ions and their analogs. J. Phys. Chem. A. 111, 4125–4143 (2007).
Limberg, C. On the trail of CrO2Cl2 and its reactions with organic compounds. Chem. Eur. J. 6, 2083–2089 (2000).
Que, Jr L. & Tolman, W. B. Biologically inspired oxidation catalysts. Nature 455, 333–340 (2008).
Fortier, S. & Hayton, T. W. Oxo ligand functionalization in the uranyl ion (UO22+). Coord. Chem. Rev. 254, 197–214 (2010).
Arnold, P. L., Love, J. B. & Patel, D. Pentavalent uranyl complexes. Coord. Chem. Rev. 253, 1973–1978 (2009).
McCleskey, T. M., Foreman, T. M., Hallman, E. E., Burns, C. J. & Sauer, N. N. Approaching zero discharge in uranium reprocessing: photochemical reduction of uranyl. Environ. Sci. Tech. 35, 547–551 (2000).
Renshaw, J. C. et al. Bioreduction of uranium: Environmental implications of a pentavalent intermediate. Environ. Sci. Tech. 39, 5657–5660 (2005).
Fox, A. R., Bart, S. C., Meyer, K. & Cummins, C. C. Towards uranium catalysis. Nature 455, 341–349 (2008).
Natrajan, L., Burdet, F., Pecaut, J. & Mazzanti, M. Synthesis and structure of a stable pentavalent-uranyl coordination polymer. J. Am. Chem. Soc. 128, 7152–7153 (2006).
Mougel, V., Horeglad, P., Nocton, G., Pecaut, J. & Mazzanti, M. Stable pentavalent uranyl species and selective assembly of a polymetallic mixed-valent uranyl complex by cation-cation interactions. Angew. Chem. Int. Ed. 48, 8477–8480 (2009).
Berthet, J. C., Siffredi, G., Thuery, P. & Ephritikhine, M. Easy access to stable pentavalent uranyl complexes. Chem. Commun. 3184–3186 (2006).
Berthet, J. C., Siffredi, G., Thuery, P. & Ephritikhine, M. Synthesis and crystal structure of pentavalent uranyl complexes. The remarkable stability of UO2X (X=I, SO3CF3) in non-aqueous solutions. Dalton Trans. 3478–3494 (2009).
Hayton, T. W. & Wu, G. Exploring the effects of reduction or Lewis acid coordination on the U=O bond of the uranyl moiety. Inorg. Chem. 48, 3065–3072 (2009).
Arnold, P. L., Patel, D., Wilson, C. & Love, J. B. Reduction and selective oxo-group silylation of the uranyl dication. Nature 451, 315–317 (2008).
Yahia, A., Arnold, P. L., Love, J. B. & Maron, L. A DFT study of the single electron reduction and silylation of the U–O bond of the uranyl dication in a macrocyclic environment. Chem. Commun. 2402 (2009).
Nocton, G. et al. Synthesis, structure, and bonding of stable complexes of pentavalent uranyl. J. Am. Chem. Soc. 132, 495–508 (2010).
Gamp, E., Edelstein, N. M., Khan Malek, C., Hubert, S. & Genet, M. Anisotropic magnetic susceptibility of single crystal UCl4 . J. Chem. Phys. 79, 2023–2026 (1983).
Lucas, R. L., Powell, D. R. & Borovik, A. S. Preparation of iron amido complexes via putative Fe(IV) imido intermediates. J. Am. Chem. Soc. 127, 11596–11597 (2005).
Zdilla, M. J., Dexheimer, J. L. & Abu-Omar, M. M. Hydrogen atom transfer reactions of imido manganese(V) corrole: one reaction with two mechanistic pathways. J. Am. Chem. Soc. 129, 11505–11511 (2007).
Bordwell, F. G., Zhang, X. & Cheng, J.-P. Comparisons of the acidities and homolytic bond dissociation energies of acidic N-H and C-H bonds in diphenylmethanes and carbazoles. J. Org. Chem. 56, 3216–3219 (1991).
Bordwell, F. G., Harrelson, Jr J. A. & Satish, A. V. Oxidation potentials of carbanions and homolytic bond dissociation energies of their conjugate acids. J. Org. Chem. 54, 3101–3105 (1989).
Renaud, P. & Fox, M. A. Electrochemical behaviour of lithium dialkylamides: the effect of aggregation. J. Am. Chem. Soc. 110, 5702–5705 (1988).
McMillen, D. F. & Golden, D. M. Hydrocarbon bond dissociation energies. Ann. Rev. Phys. Chem. 33, 493–532 (1982).
Kasai, P. H. & McLeod, D. Electron spin resonance study of heterocycles. II. Pyrrole, pyrazole, imidazole, and indole anion radicals. J. Am. Chem. Soc. 95, 27–31 (1973).
Reid, S. D., Wilson, C., Blake, A. J. & Love, J. B. Tautomerisation and hydrogen-bonding interactions in four-coordinate metal halide and azide complexes of N-donor-extended dipyrromethanes. Dalton Trans. 39, 418–425 (2010).
Büttner, T. et al. A stable aminyl radical metal complex. Science 307, 235–238 (2005).
Jørgensen, C. K. & Reisfeld, R. Uranyl photophysics. Struct. Bond. 50, 121–171 (1982).
McCleskey, T. M., Burns, C. J. & Tumas, W. Uranyl photochemistry with alkenes: Distinguishing between H-atom abstraction and electron transfer. Inorg. Chem. 38, 5924–5925 (1999).
Acknowledgements
We thank the EPSRC(UK), EaStCHEM, the University of Edinburgh and the CEA, CNRS and UPS for support. L.M. is grateful to Institut Universitaire de France. CalMip (CNRS, Toulouse, France), CINES (CNRS, Montpellier, France) and CCRT (CEA, France) are acknowledged for calculation facilities. The authors are grateful to D. Graham, I. Lamour, R. E. Mulvey and S. Robertson for help with obtaining the Raman spectroscopic measurements.
Author information
Authors and Affiliations
Contributions
A.-F.P. and E.H. synthesized and characterized the compounds, and solved the crystal structure data; A.Y. and L.M. carried out the DFT calculations; and S.J.P. solved and refined the disorder components for the crystal structure of 3py. P.L.A. and J.B.L. generated and managed the project, helped characterize the compounds, analysed the data and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 5799 kb)
Supplementary information
1thf (GIF 1968 kb)
Supplementary information
3py (GIF 3405 kb)
Supplementary information
5thf (GIF 1976 kb)
Supplementary information
Crystallographic data for compound 2thf (CIF 50 kb)
Supplementary information
Crystallographic data for compound 3py (CIF 91 kb)
Supplementary information
Crystallographic data for compound 4py (CIF 68 kb)
Supplementary information
Crystallographic data for compound 5thf (CIF 79 kb)
Rights and permissions
About this article
Cite this article
Arnold, P., Pécharman, AF., Hollis, E. et al. Uranyl oxo activation and functionalization by metal cation coordination. Nature Chem 2, 1056–1061 (2010). https://doi.org/10.1038/nchem.904
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.904
This article is cited by
-
Gas-Phase Deconstruction of UO22+: Mass Spectrometry Evidence for Generation of [OUVICH]+ by Collision-Induced Dissociation of [UVIO2(C≡CH)]+
Journal of the American Society for Mass Spectrometry (2019)
-
A computational investigation of polypyrrolic macrocyclic actinyl complexes: effects of explicit solvent coordination on structure, vibrational spectra and redox property
Theoretical Chemistry Accounts (2016)
-
Structural/electronic properties and reaction energies of a series of mono- and bis-uranyl dihalides equatorially coordinated by N/O ligands
Journal of Molecular Modeling (2014)
-
Strongly coupled binuclear uranium–oxo complexes from uranyl oxo rearrangement and reductive silylation
Nature Chemistry (2012)
-
Uranium and manganese assembled in a wheel-shaped nanoscale single-molecule magnet with high spin-reversal barrier
Nature Chemistry (2012)