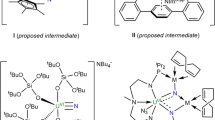
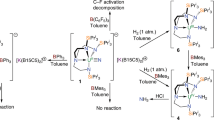
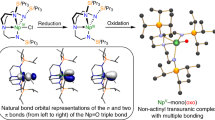
Abstract
Uranium nitride [U≡N]x is an alternative nuclear fuel that has great potential in the expanding future of nuclear power; however, very little is known about the U≡N functionality. We show, for the first time, that a terminal uranium nitride complex can be generated by photolysis of an azide (U–N=N=N) precursor. The transient U≡N fragment is reactive and undergoes insertion into a ligand C–H bond to generate new N–H and N–C bonds. The mechanism of this unprecedented reaction has been evaluated through computational and spectroscopic studies, which reveal that the photochemical azide activation pathway can be shut down through coordination of the terminal azide ligand to the Lewis acid B(C6F5)3. These studies demonstrate that photochemistry can be a powerful tool for inducing redox transformations for organometallic actinide complexes, and that the terminal uranium nitride fragment is reactive, cleaving strong C–H bonds.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Yeamans, C. B. et al. Oxidative ammonolysis of uranium(IV) fluorides to uranium(VI) nitride. J. Nucl. Mater. 374, 75–78 (2008).
Streit, M. & Ingold, F. Nitrides as a nuclear fuel option. J. Eur. Ceram. Soc. 25, 2687–2692 (2005).
Denning, R. G. Electronic structure and bonding in actinyl ions and their analogs. J. Phys. Chem. A 111, 4125–4143 (2007).
Arnold, P. L. et al. Reduction and selective oxo group silylation of the uranyl dication. Nature 451, 315–317 (2008).
Burdet, F., Pecaut, J. & Mazzanti, M. Isolation of a tetrameric cation–cation complex of pentavalent uranyl. J. Am. Chem. Soc. 128, 16512–16513 (2006).
Steele, H. & Taylor, R. J. A theoretical study of the inner-sphere disproportionation reaction mechanism of the pentavalent actinyl ions. Inorg. Chem. 46, 6311–6318 (2007).
Korobkov, I., Gambarotta, S. & Yap, G. P. A. A highly reactive uranium complex supported by the calix[4]tetrapyrrole tetraanion affording dinitrogen cleavage, solvent deoxygenation, and polysilanol depolymerization. Angew. Chem. Int. Ed. 41, 3433–3436 (2002).
Evans, W. J., Kozimor, S. A. & Ziller, J. W. Molecular octa-uranium rings with alternating nitride and azide bridges. Science 309, 1835–1838 (2005).
Evans, W. J. et al. Analysis of uranium azide and nitride complexes by atmospheric pressure chemical ionization mass spectrometry. Inorg. Chem. 46, 8008–8018 (2007).
Fox, A. R. & Cummins, C. C. Uranium–nitrogen multiple bonding: the case of a four-coordinate uranium(VI) nitridoborate complex. J. Am. Chem. Soc. 131, 5716–5717 (2009).
Fox, A. R., Arnold, P. L. & Cummins, C. C. Uranium–nitrogen multiple bonding: isostructural anionic, neutral and cationic uranium nitride complexes featuring a linear U=N=U core. J. Am. Chem. Soc. 132, 3250–3251 (2010).
Nocton, G., Pecaut, J. & Mazzanti, M. A nitrido-centered uranium azido cluster obtained from a uranium azide. Angew. Chem. Int. Ed. 47, 3040–3042 (2008).
Berry, J. F. et al. An octahedral coordination complex of iron(VI). Science 312, 1937–1941 (2006).
Vogel, C. et al. An iron nitride complex. Angew. Chem. Int. Ed. 47, 2681–2684 (2008).
Scepaniak, J. J. et al. Structural and spectroscopic characterization of an electrophilic iron nitrido complex. J. Am. Chem. Soc. 130, 10515–10517 (2008).
Scepaniak, J. J. et al. Formation of ammonia from an iron nitrido complex. Angew. Chem. Int. Ed. 48, 3158–3160 (2009).
Kalina, D. G., Marks, T. J. & Wachter, W. A. Photochemical synthesis of low-valent organothorium complexes. Evidence for photoinduced β-hydride elimination. J. Am. Chem. Soc. 99, 3877–3879 (1977).
Bruno, J. W. et al. Mechanistic study of photoinduced β-hydride elimination. The facile photochemical synthesis of low-valent thorium and uranium organometallics. J. Am. Chem. Soc. 104, 1860–1869 (1982).
Shaik, S. et al. Theoretical perspective on the structure and mechanism of cytochrome P450 enzymes. Chem. Rev. 105, 2279–2328 (2005).
Thomson, R. K. et al. Noble reactions for the actinides: safe gold-based access to organouranium and azido complexes. Eur. J. Inorg. Chem. 1451–1455 (2009).
Dori, Z. & Ziolo, R. F. Chemistry of coordinated azides. Chem. Rev. 73, 247–254 (1973).
Rozsnyai, L. F. & Wrighton, M. S. Selective electrochemical deposition of polyaniline via photopatterning of a monolayer-modified substrate. J. Am. Chem. Soc. 116, 5993–5994 (1994).
Arney, D. S. J. & Burns, C. J. Synthesis and properties of high-valent organouranium complexes containing terminal organoimido and oxo functional groups. A new class of organo-f-element complexes. J. Am. Chem. Soc. 117, 9448–9460 (1995).
Peters, R. G., Warner, B. P. & Burns, C. J. The catalytic reduction of azides and hydrazines using high-valent organouranium complexes. J. Am. Chem. Soc. 121, 5585–5586 (1999).
Peters, R. G. et al. C–H bond activation with actinides: the first example of intramolecular ring bite of a pentamethylcyclopentadienyl methyl group. Organometallics 18, 2587–2589 (1999).
Zi, G. et al. Preparation and reactions of base-free bis(1,2,4-tri-tert-butylcyclopentadienyl)uranium oxide, Cp'2UO. Organometallics 24, 4251–4264 (2005).
Monreal, M. J. & Diaconescu, P. L. A weak interaction between iron and uranium in uranium alkyl complexes supported by ferrocene diamide ligands. Organometallics 27, 1702–1706 (2008).
Fraenk, W. et al. Pentafluorophenyl and phenyl substituted azidoborates. Can. J. Chem. 80, 1444–1450 (2002).
Crevier, T. J. & Mayer, J. M. Insertion of an osmium nitride into boron–carbon bonds. Angew. Chem. Int. Ed. 37, 1891–1893 (1998).
Evans, W. J. et al. A crystallizable f-element tuck-in complex: the tuck-in tuck-over uranium metallocene [(C5Me5)U{μ-η5:η1:η1-C5Me3(CH2)2}(μ-H)2U(C5Me5)2]. Angew. Chem. Int. Ed. 47, 5075–5078 (2008).
Gardner, B. M. et al. A crystallizable dinuclear tuck-in-tuck-over tuck-over dialkyl tren uranium complex and double dearylation of BPh4− to give the BPh2-functionalized metallocycle [U{N(CH2CH2NSiMe3)2(CH2CH2NSiMe2CHBPh2)}(THF)]. J. Am. Chem. Soc. 131, 10388–10389 (2009).
Arney, D. S. J., Burns, C. J. & Smith, D. C. Synthesis and structure of the first uranium(VI) organometallic complex. J. Am. Chem. Soc. 114, 10068–10069 (1992).
Hayton, T. W. Metal–ligand multiple bonding in uranium: structure and reactivity. Dalton Trans. 39, 1145–1158 (2010).
Acknowledgements
The authors thank the Los Alamos National Laboratory (LANL) G. T. Seaborg Institute for Transactinium Science for a postdoctoral fellowship to R.K.T., LANL for a Director's postdoctoral fellowship to T.C., and the Division of Chemical Sciences, Office of Basic Energy Science, Heavy Element Chemistry program and the LANL Laboratory Directed Research and Development (LDRD) program for funding. R. M. Chamberlin and D. L. Clark (both LANL) are thanked for helpful discussions.
Author information
Authors and Affiliations
Contributions
R.K.T. synthesized and characterized the compounds and wrote the manuscript. B.L.S. collected single-crystal X-ray crystallographic data and solved the structures. T.C. and E.R.B. performed DFT calculations. D.E.M. aided in the analysis and interpretation of UV–vis–NIR spectral data. J.L.K. generated and managed the project and helped write the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 1656 kb)
Supplementary information
Crystallographic data for compound 2 (CIF 16 kb)
Supplementary information
Crystallographic data for compound 3 (CIF 28 kb)
Supplementary information
Crystallographic data for compound 4 (CIF 18 kb)
Supplementary information
Crystallographic data for compound 5 (CIF 29 kb)
Supplementary information
Crystallographic data for compound 7 (CIF 25 kb)
Rights and permissions
About this article
Cite this article
Thomson, R., Cantat, T., Scott, B. et al. Uranium azide photolysis results in C–H bond activation and provides evidence for a terminal uranium nitride. Nature Chem 2, 723–729 (2010). https://doi.org/10.1038/nchem.705
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.705
This article is cited by
-
Photochemical Synthesis of Transition Metal-Stabilized Uranium(VI) Nitride Complexes
Nature Communications (2022)
-
Charge frustration in ligand design and functional group transfer
Nature Reviews Chemistry (2021)
-
A platinum(ii) metallonitrene with a triplet ground state
Nature Chemistry (2020)
-
Terminal uranium(V)-nitride hydrogenations involving direct addition or Frustrated Lewis Pair mechanisms
Nature Communications (2020)
-
Transition-metal-bridged bimetallic clusters with multiple uranium–metal bonds
Nature Chemistry (2019)