Abstract
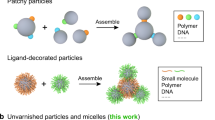
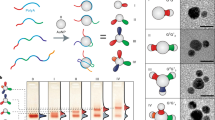
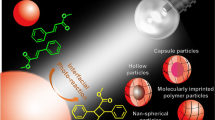
As colloidal self-assembly increasingly approaches the complexity of natural systems, an ongoing challenge is to generate non-centrosymmetric structures. For example, patchy, Janus or living crystallization particles have significantly advanced the area of polymer assembly. It has remained difficult, however, to devise polymer particles that associate in a directional manner, with controlled valency and recognition motifs. Here, we present a method to transfer DNA patterns from a DNA cage to a polymeric nanoparticle encapsulated inside the cage in three dimensions. The resulting DNA-imprinted particles (DIPs), which are ‘moulded’ on the inside of the DNA cage, consist of a monodisperse crosslinked polymer core with a predetermined pattern of different DNA strands covalently ‘printed’ on their exterior, and further assemble with programmability and directionality. The number, orientation and sequence of DNA strands grafted onto the polymeric core can be controlled during the process, and the strands are addressable independently of each other.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Seeman, N. C. DNA in a material world. Nature 421, 427–431 (2003).
Aldaye, F. A., Palmer, A. L. & Sleiman, H. F. Assembling materials with DNA as the guide. Science 321, 1795–1799 (2008).
Seeman, N. C. DNA engineering and its application to nanotechnology. Trends Biotechnol. 17, 437–443 (1999).
Rothemund, P. W. K. Folding DNA to create nanoscale shapes and patterns. Nature 440, 297–302 (2006).
Aldaye, F. A. & Sleiman, H. F. Modular access to structurally switchable 3D discrete DNA assemblies. J. Am. Chem. Soc. 129, 13376–13377 (2007).
He, Y. et al. Hierarchical self-assembly of DNA into symmetric supramolecular polyhedra. Nature 452, 198–201 (2008).
Ke, Y., Ong, L. L., Shih, W. M. & Yin, P. Three-dimensional structures self-assembled from DNA bricks. Science 338, 1177–1183 (2012).
Winfree, E., Liu, F., Wenzler, L. A. & Seeman, N. C. Design and self-assembly of two-dimensional DNA crystals. Nature 394, 539–544 (1998).
Benson, E. et al. DNA rendering of polyhedral meshes at the nanoscale. Nature 523, 441–444 (2015).
Douglas, S. M. et al. Self-assembly of DNA into nanoscale three-dimensional shapes. Nature 459, 414–418 (2009).
Ke, Y. et al. Scaffolded DNA origami of a DNA tetrahedron molecular container. Nano Lett. 9, 2445–2447 (2009).
Kwak, M. & Herrmann, A. Nucleic acid/organic polymer hybrid materials: synthesis, superstructures, and applications. Angew. Chem. Int. Ed. 49, 8574–8587 (2010).
Alemdaroglu, F. E. & Herrmann, A. DNA meets synthetic polymers—highly versatile hybrid materials. Org. Biomol. Chem. 5, 1311–1320 (2007).
Yang, C. J., Pinto, M., Schanze, K. & Tan, W. Direct synthesis of an oligonucleotide–poly(phenylene ethynylene) conjugate with a precise one-to-one molecular ratio. Angew. Chem. Int. Ed. 117, 2628–2632 (2005).
Jeong, J. H. & Park, T. G. Novel polymer−DNA hybrid polymeric micelles composed of hydrophobic poly(D,L-lactic-co-glycolic acid) and hydrophilic oligonucleotides. Bioconj. Chem. 12, 917–923 (2001).
Tan, X. et al. Light-triggered, self-immolative nucleic acid–drug nanostructures. J. Am. Chem. Soc. 137, 6112–6115 (2015).
Kwak, M. & Herrmann, A. Nucleic acid amphiphiles: synthesis and self-assembled nanostructures. Chem. Soc. Rev. 40, 5745–5755 (2011).
Edwardson, T. G. W., Carneiro, K. M. M., Serpell, C. J. & Sleiman, H. F. An efficient and modular route to sequence-defined polymers appended to DNA. Angew. Chem. Int. Ed. 53, 4567–4571 (2014).
Albert, S. K. et al. Self-assembly of DNA–oligo(p-phenylene-ethynylene) hybrid amphiphiles into surface-engineered vesicles with enhanced emission. Angew. Chem. Int. Ed. 53, 8352–8357 (2014).
Jiang, S. et al. Janus particle synthesis and assembly. Adv. Mater. 22, 1060–1071 (2010).
Wurm, F. & Kilbinger, A. F. M. Polymeric Janus particles. Angew. Chem. Int. Ed. 48, 8412–8421 (2009).
Roh, K.-H., Martin, D. C. & Lahann, J. Biphasic Janus particles with nanoscale anisotropy. Nat. Mater. 4, 759–763 (2005).
Badi, N. & Lutz, J.-F. Sequence control in polymer synthesis. Chem. Soc. Rev. 38, 3383–3390 (2009).
Lutz, J.-F., Ouchi, M., Liu, D. R. & Sawamoto, M. Sequence-controlled polymers. Science 341, 1238149 (2013).
Qiu, H. et al. Uniform patchy and hollow rectangular platelet micelles from crystallizable polymer blends. Science 352, 697–701 (2016).
Walther, A. & Müller, A. H. E. Janus particles: synthesis, self-assembly, physical properties, and applications. Chem. Rev. 113, 5194–5261 (2013).
Suzuki, K., Hosokawa, K. & Maeda, M. Controlling the number and positions of oligonucleotides on gold nanoparticle surfaces. J. Am. Chem. Soc. 131, 7518–7519 (2009).
Kim, J.-W., Kim, J.-H. & Deaton, R. DNA-linked nanoparticle building blocks for programmable matter. Angew. Chem. Int. Ed. 50, 9185–9190 (2011).
Zanchet, D., Micheel, C. M., Parak, W. J., Gerion, D. & Alivisatos, A. P. Electrophoretic isolation of discrete Au nanocrystal/DNA conjugates. Nano Lett. 1, 32–35 (2001).
Zhao, Z., Jacovetty, E. L., Liu, Y. & Yan, H. Encapsulation of gold nanoparticles in a DNA origami cage. Angew. Chem. Int. Ed. 50, 2041–2044 (2011).
Li, Y., Liu, Z., Yu, G., Jiang, W. & Mao, C. Self-assembly of molecule-like nanoparticle clusters directed by DNA nanocages. J. Am. Chem. Soc. 137, 4320–4323 (2015).
Wang, Y. et al. Colloids with valence and specific directional bonding. Nature 491, 51–55 (2012).
Xu, X., Rosi, N. L., Wang, Y., Huo, F. & Mirkin, C. A. Asymmetric functionalization of gold nanoparticles with oligonucleotides. J. Am. Chem. Soc. 128, 9286–9287 (2006).
Li, Z. et al. Improving the yield of mono-DNA-functionalized gold nanoparticles through dual steric hindrance. J. Am. Chem. Soc. 133, 15284–15287 (2011).
Pei, H. et al. Designed diblock oligonucleotide for the synthesis of spatially isolated and highly hybridizable functionalization of DNA–gold nanoparticle nanoconjugates. J. Am. Chem. Soc. 134, 11876–11879 (2012).
Tan, S. J., Campolongo, M. J., Luo, D. & Cheng, W. Building plasmonic nanostructures with DNA. Nat. Nanotech. 6, 268–276 (2011).
Maye, M. M., Nykypanchuk, D., Cuisinier, M., van der Lelie, D. & Gang, O. Stepwise surface encoding for high-throughput assembly of nanoclusters. Nat. Mater. 8, 388–391 (2009).
Tan, L. H., Xing, H., Chen, H. & Lu, Y. Facile and efficient preparation of anisotropic DNA-functionalized gold nanoparticles and their regioselective assembly. J. Am. Chem. Soc. 135, 17675–17678 (2013).
Tian, Y. et al. Prescribed nanoparticle cluster architectures and low-dimensional arrays built using octahedral DNA origami frames. Nat. Nanotech. 10, 637–644 (2015).
Schreiber, R., Santiago, I., Ardavan, A. & Turberfield, A. J. Ordering gold nanoparticles with DNA origami nanoflowers. ACS Nano 10, 7303–7306 (2016).
Liu, W., Halverson, J., Tian, Y., Tkachenko, A. V. & Gang, O. Self-organized architectures from assorted DNA-framed nanoparticles. Nat. Chem. 8, 867–873 (2016).
Edwardson, T. G. W., Lau, K. L., Bousmail, D., Serpell, C. J. & Sleiman, H. F. Transfer of molecular recognition information from DNA nanostructures to gold nanoparticles. Nat. Chem. 8, 162–170 (2016).
Zhang, Y. et al. Transfer of two-dimensional oligonucleotide patterns onto stereocontrolled plasmonic nanostructures through DNA-origami-based nanoimprinting lithography. Angew. Chem. Int. Ed. 55, 8036–8040 (2016).
Edwardson, T. G. W., Carneiro, K. M. M., McLaughlin, C. K., Serpell, C. J. & Sleiman, H. F. Site-specific positioning of dendritic alkyl chains on DNA cages enables their geometry-dependent self-assembly. Nat. Chem. 5, 868–875 (2013).
Serpell, C. J., Edwardson, T. G. W., Chidchob, P., Carneiro, K. M. M. & Sleiman, H. F. Precision polymers and 3D DNA nanostructures: emergent assemblies from new parameter space. J. Am. Chem. Soc. 136, 15767–15774 (2014).
Chidchob, P., Edwardson, T. G. W., Serpell, C. J. & Sleiman, H. F. Synergy of two assembly languages in DNA nanostructures: self-assembly of sequence-defined polymers on DNA cages. J. Am. Chem. Soc. 138, 4416–4425 (2016).
Trinh, T., Chidchob, P., Bazzi, H. S. & Sleiman, H. F. DNA micelles as nanoreactors: efficient DNA functionalization with hydrophobic organic molecules. Chem. Commun. 52, 10914–10917 (2016).
Liao, C. et al. Melittin aggregation in aqueous solutions: insight from molecular dynamics simulations. J. Phys. Chem. B 119, 10390–10398 (2015).
Macfarlane, R. J., O'Brien, M. N., Petrosko, S. H. & Mirkin, C. A. Nucleic acid-modified nanostructures as programmable atom equivalents: forging a new ‘table of elements’. Angew. Chem. Int. Ed. 52, 5688–5698 (2013).
Jones, M. R., Seeman, N. C. & Mirkin, C. A. Programmable materials and the nature of the DNA bond. Science 347, 1260901 (2015).
Lee, J. H., Yigit, M. V., Mazumdar, D. & Lu, Y. Molecular diagnostic and drug delivery agents based on aptamer-nanomaterial conjugates. Adv. Drug Deliv. Rev. 62, 592–605 (2010).
Acknowledgements
The authors acknowledge the Natural Sciences and Engineering Research Council of Canada (NSERC), the Canadian Institutes for Health Research, the Centre for Self-Assembled Chemical Structures (CSACS), the Qatar Research Foundation (project no. NPRP 5-1505-1-250) and the Canada Research Chairs Program for financial support. H.F.S. is a Cottrell Scholar of the Research Corporation.
Author information
Authors and Affiliations
Contributions
H.F.S. and T.T. designed the project. T.T. mainly contributed to the production of experimental results. C.L. and J.L. performed computer modelling molecular dynamics simulations of the cube/DNA-polymer. M.B., H.S.B. and V.T. synthesized the hydrophobic unit and its phosphoramidite derivative. All authors agreed to all the content of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 4720 kb)
Supplementary information
Supplementary Movie 1 (MP4 29703 kb)
Rights and permissions
About this article
Cite this article
Trinh, T., Liao, C., Toader, V. et al. DNA-imprinted polymer nanoparticles with monodispersity and prescribed DNA-strand patterns. Nature Chem 10, 184–192 (2018). https://doi.org/10.1038/nchem.2893
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.2893
This article is cited by
-
A sonosensitiser‐based polymeric nanoplatform for chemo‐sonodynamic combination therapy of lung cancer
Journal of Nanobiotechnology (2021)
-
Valence-programmable nanoparticle architectures
Nature Communications (2020)
-
Complex assemblies and crystals guided by DNA
Nature Materials (2020)
-
Chemical reactivity under nanoconfinement
Nature Nanotechnology (2020)
-
Oligonucleotide–Polymer Conjugates: From Molecular Basics to Practical Application
Topics in Current Chemistry (2020)