Abstract
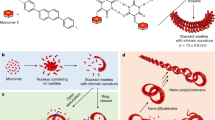
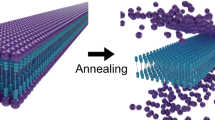
Despite recent advances in the synthesis of increasingly complex topologies at the molecular level, nano- and microscopic weaves have remained difficult to achieve. Only a few diaxial molecular weaves exist—these were achieved by templation with metals. Here, we present an extended triaxial supramolecular weave that consists of self-assembled organic threads. Each thread is formed by the self-assembly of a building block comprising a rigid oligoproline segment with two perylene-monoimide chromophores spaced at 18 Å. Upon π stacking of the chromophores, threads form that feature alternating up- and down-facing voids at regular distances. These voids accommodate incoming building blocks and establish crossing points through CH–π interactions on further assembly of the threads into a triaxial woven superstructure. The resulting micrometre-scale supramolecular weave proved to be more robust than non-woven self-assemblies of the same building block. The uniform hexagonal pores of the interwoven network were able to host iridium nanoparticles, which may be of interest for practical applications.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Soffer, O., Adovasio, J. M. & Hyland, D. C. in Enduring Records: The Environmental and Cultural Heritage of Wetlands (ed. Purdy, B. A.) 233–245 (Oxbow, 2001).
Tyler, T. in Specialist Yarn and Fabric Structures: Developments and Applications (ed. Gong, R. H.) Ch. 7 (Woodhead, 2011).
Aida, T., Meijer, E. W. & Stupp, S. I. Functional supramolecular polymers. Science 335, 813–817 (2012).
Korevaar, P. A. et al. Pathway complexity in supramolecular polymerization. Nature 481, 492–496 (2012).
Yu, Z. et al. Simultaneous covalent and non-covalent hybrid polymerizations. Science 351, 497–502 (2016).
Han, D. et al. DNA origami with complex curvatures in three-dimensional space. Science 332, 342–346 (2011).
Malo, J. et al. Engineering a 2D protein–DNA crystal. Angew. Chem. Int. Ed. 44, 3057–3061 (2005).
Ponnuswamy, N., Cougnon, F. B. L., Clough, J. M., Pantoş, G. D. & Sanders, J. K. M. Discovery of an organic trefoil knot. Science 338, 783–785 (2012).
Chichak, K. S. et al. Molecular borromean rings. Science 304, 1308–1312 (2004).
Danon, J. J. et al. Braiding a molecular knot with eight crossings. Science 355, 159–162 (2017).
Gil-Ramírez, G., Leigh, D. A. & Stephens, A. J. Catenanes: fifty years of molecular links. Angew. Chem. Int. Ed. 54, 6110–6150 (2015).
Van Calcar, P. M., Olmstead, M. M. & Balch, A. L. Construction of a knitted crystalline polymer through the use of gold(I)–gold(I) interactions. J. Chem. Soc. Chem. Commun. 1773–1774 (1995).
Axtell, E. A. III, Liao, J.-H. & Kanatzidis, M. G. Flux synthesis of LiAuS and NaAuS: ‘chicken-wire-like’ layer formation by interweaving of (AuS)nn− threads. Comparison with α-HgS and AAuS (A = K, Rb). Inorg. Chem. 37, 5583–5587 (1998).
Carlucci, L., Ciani, G., Gramaccioli, A., Proserpio, D. M. & Rizzato, S. Crystal engineering of coordination polymers and architectures using the [Cu(2,2′-bipy)]2+ molecular corner as building block (bipy = 2,2′-bipyridyl). Cryst. Eng. Commun. 29, 1–10 (2000).
Ino, I. et al. 2-D interwoven and 3-D 5-fold interpenetrating silver(I) complexes of 1-(isocyanidomethyl)-1H-benzotriazole and 1,3-bis(dicyanomethylidene)indan. Inorg. Chem. 39, 4273–4279 (2000).
Bu, X.-H. et al. A spontaneously resolved chiral molecular box: a cyclic tetranuclear Zn(II) complex with DPTZ (DPTZ = 3,6-di-2-pyridyl-1,2,4,5-tetrazine). Chem. Commun. 971–972 (2000).
Bark, T., Dîggeli, M., Stoeckli-Evans, H. & von Zelewsky, A. Designed molecules for self-assembly: the controlled formation of two chiral self-assembled polynuclear species with predetermined configuration. Angew. Chem. Int. Ed. 40, 2848–2851 (2001).
Hausmann, J. & Brooker, S. Control of molecular architecture by use of the appropriate ligand isomer: a mononuclear ‘corner-type’ versus a tetranuclear [2 × 2] grid-type cobalt(III) complex. Chem. Commun. 1530–1531 (2004).
Beves, J. E., Danon, J. J., Leigh, D. A., Lemonnier, J.-F. & Vitorica-Yrezabal, I. J. A Solomon link through an interwoven molecular grid. Angew. Chem. Int. Ed. 54, 7555–7559 (2015).
Payamyar, P., King, B. T., Öttinger, H. C. & Schlüter, A. D. Two-dimensional polymers: concepts and perspectives. Chem. Commun. 52, 18–34 (2016).
Liu, Y. et al. Weaving of organic threads into a crystalline covalent organic framework. Science 351, 365–369 (2016).
Wang, Z. et al. Molecular weaving via surface-templated epitaxy of crystalline coordination networks. Nat. Commun. 8, 14442.
Kümin, M., Sonntag, L. S. & Wennemers, H. Azidoproline containing helices: stabilization of the polyproline II structure by a functionalizable group. J. Am. Chem. Soc. 129, 466–467 (2007).
Wilhelm, P., Lewandowski, B., Trapp, N. & Wennemers, H. A crystal structure of an oligoproline PPII-helix, at last. J. Am. Chem. Soc. 136, 15829–15832 (2014).
Weingarten, A. S. et al. Self-assembling hydrogel scaffolds for photocatalytic hydrogen production. Nat. Chem. 6, 964–970 (2014).
Lewandowska, U. et al. Hierarchical supramolecular assembly of sterically demanding π-systems by conjugation with oligoprolines. Angew. Chem. Int. Ed. 53, 12537–12541 (2014).
Lewandowska, U. et al. Effect of structural modifications on the self-assembly of oligoprolines conjugated with sterically demanding chromophores. Chem. Eur. J. 22, 3804–3809 (2016).
Meyer, E. A., Castellano, R. K. & Diederich, F. Interactions with aromatic rings in chemical and biological recognition. Angew. Chem. Int. Ed. 42, 1210–1250 (2003).
Nishio, M. The CH/π hydrogen bond in chemistry. Conformation, supramolecules, optical resolution and interactions involving carbohydrates. Phys. Chem. Chem. Phys. 13, 13873–13900 (2011).
Plevin, M. J., Bryce, D. L. & Boisbouvier, J. Direct detection of CH/π interactions in proteins. Nat. Chem. 2, 466–471 (2010).
Frischmann, P. D. & Würthner, F. Synthesis of a non-aggregating bay-unsubstituted perylene bisimide dye with latent bromo groups for C–C cross coupling. Org. Lett. 15, 4674–4677 (2013).
Chen, Q., Chul Bae, S. & Granick, S. Directed self-assembly of a colloidal kagome lattice. Nature 469, 381–384 (2011).
Jonkheijm, P., van der Schoot, P., Schenning, A. P. H. J. & Meijer, E. W. Probing the solvent assisted nucleation pathway in chemical self-assembly. Science 313, 80–83 (2006).
Rueping, M. et al. Size-selective, stabilizer-free hydrogenolytic synthesis of iridium nanoparticles supported on carbon nanotubes. Chem. Mater. 23, 2008–2010 (2011).
Acknowledgements
The authors acknowledge the VW Foundation for financial support and the ETH Scientific Center for Optical and Electron Microscopy (ScopeM) for support. The authors also acknowledge Beamline 9 of the DELTA electron storage ring in Dortmund for providing synchrotron radiation and technical support for GIWAXS measurements. The authors thank D. Schlüter for discussions and J. Schnabl for the illustration of the supramolecular weave.
Author information
Authors and Affiliations
Contributions
U.L., W.Z., S.C., J.T. and H.W. conceived the molecular design and elucidated the structure. U.L., W.Z., S.C., J.T., R.B., E.M.B., S.S., K.W., N.A.K.O., R.S. and W.P. designed and carried out the experimental work. U.L., S.C. and H.W. wrote the manuscript with major contributions from R.B., W.Z., W.P. and K.M., as well as input from all other authors. All authors contributed to analysis of the data. K.M. and H.W. directed the research and conceived the overall project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 15110 kb)
Supplementary information
Supplementary information (MP4 2180 kb)
Rights and permissions
About this article
Cite this article
Lewandowska, U., Zajaczkowski, W., Corra, S. et al. A triaxial supramolecular weave. Nature Chem 9, 1068–1072 (2017). https://doi.org/10.1038/nchem.2823
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.2823
This article is cited by
-
Folk arts-inspired twice-coagulated configuration-editable tough aerogels enabled by transformable gel precursors
Nature Communications (2023)
-
Molecular weaving
Nature Materials (2022)
-
Shape-assisted self-assembly
Nature Communications (2022)
-
A molecular endless (74) knot
Nature Chemistry (2021)
-
Self-assembly of a layered two-dimensional molecularly woven fabric
Nature (2020)