Abstract
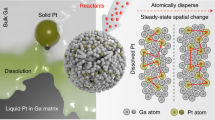
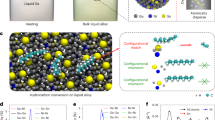
A strategy to develop improved catalysts is to create systems that merge the advantages of heterogeneous and molecular catalysis. One such system involves supported liquid-phase catalysts, which feature a molecularly defined, catalytically active liquid film/droplet layer adsorbed on a porous solid support. In the past decade, this concept has also been extended to supported ionic liquid-phase catalysts. Here we develop this idea further and describe supported catalytically active liquid metal solutions (SCALMS). We report a liquid mixture of gallium and palladium deposited on porous glass that forms an active catalyst for alkane dehydrogenation that is resistant to coke formation and is thus highly stable. X-ray diffraction and X-ray photoelectron spectroscopy, supported by theoretical calculations, confirm the liquid state of the catalytic phase under the reaction conditions. Unlike traditional heterogeneous catalysts, the supported liquid metal reported here is highly dynamic and catalysis does not proceed at the surface of the metal nanoparticles, but presumably at homogeneously distributed metal atoms at the surface of a liquid metallic phase.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Acres, G. J. K., Bond, G. C., Cooper, B. J. & Dawson, J. A. Use of supported solutions of rhodium trichloride for homogeneous catalysis. J. Catal. 6, 139–141 (1966).
Arhancet, J. P., Davis, M. E., Merola, J. S. & Hanson, B. E. Hydroformylation by supported aqueous-phase catalysis: a new class of heterogeneous catalysts. Nature 339, 454–455 (1989).
Zhao, F., Fujita, S. & Arai, M. Developments and applications of supported liquid phase. Curr. Org. Chem. 10, 1681–1695 (2006).
Mehnert, C. P., Cook, R. A., Dispenziere, N. C. & Afeworki, M. Supported ionic liquid catalysis—a new concept for homogeneous hydroformylation catalysis. J. Am. Chem. Soc. 124, 12932–12933 (2003).
Jakuttis, M. et al. Rhodium-phosphite SILP catalysis for the highly selective hydroformylation of mixed C4 feedstocks. Ang. Chem. Int. Ed. 50, 4492–4495 (2011).
Riisager, A., Jørgensen, B., Wasserscheid, P. & Fehrmann, R. First application of supported ionic liquid phase (SILP) catalysis for continuous methanol carbonylation. Chem. Commun. 9, 994–996 (2006).
Ruta, M., Yuranov, I., Dyson, P. J., Laurenczy, G. & Kiwi-Minsker, L. Structured fiber supports for ionic liquid-phase catalysis used in gas-phase continuous hydrogenation. J. Catal. 247, 269–276 (2007).
Schneider, M. J., Lijewski, M., Wölfel, R., Haumann, M. & Wasserscheid, P. Continuous gas-phase hydroaminomethylation using supported ionic liquid phase catalysts. Ang. Chem. Int. Ed. 52, 6996–6999 (2013).
Steinrück, H.-P. & Wasserscheid, P. Ionic liquids in catalysis. Cat. Lett. 145, 380–397 (2015).
Abai, M. et al. An ionic liquid process for mercury removal from natural gas. Dalton Trans. 44, 8617–8624 (2015).
Earle, M. J. et al. The distillation and volatility of ionic liquids. Nature 439, 831–834 (2006).
Maton, C., de Vos, N. & Stevens, C. V. Ionic liquid thermal stabilities: decomposition mechanisms and analysis tools. Chem. Soc. Rev. 42, 5963–5977 (2013).
Liu, J. Catalysis by supported single metal atoms. ACS Catal. 7, 34–59 (2017).
Furukawa, S. & Komatsu, T. Intermetallic compounds: promising inorganic materials for well-structured and electronically modified reaction environments for efficient catalysis. ACS Catal. 7, 735–765 (2017).
Yatsenko, P. S., Sabirzyanov, N. A. & Yatsenko, A. S. Dissolution rates and solubility of some metals in liquid gallium and aluminum. J. Physic Conf. Ser. 98, 1–7 (2008).
Föttinger, K. & Rupprechter, G. In situ spectroscopy of complex surface reactions on supported Pd–Zn, Pd–Ga, and Pd(Pt)–Cu nanoparticles. Acc. Chem. Res. 47, 3071–33079 (2014).
Armbrüster, M. et al. How to control the selectivity of palladium-based catalysts in hydrogenation reactions: the role of subsurface chemistry. ChemCatChem 4, 1048–1063 (2012).
Prinz, J. et al. Adsorption of small hydrocarbons on the three-fold PdGa surfaces: the road to selective hydrogenation. J. Am. Chem. Soc. 136, 11792–11798 (2014).
Lorenz, H. et al. Methanol steam reforming: CO2-selective Pd2Ga phases supported on α- and γ-Ga2O3 . Appl. Cat. A 453, 34–44 (2013).
Okamoto, H. Ga–Pd (gallium–palladium). J. Phase Equilib. Diffus. 29, 466–467 (2008).
Carrá, S. & Forni, L. Catalytic dehydrogenation of C4 hydrocarbons over chromia-alumina. Cat. Rev. 5, 159–198 (1972).
Sattler, J. J. H. B., Ruiz-Martinez, J., Santillan-Jimenez, E. & Weckhuysen, B. M. Catalytic dehydrogenation of light alkanes on metals and metal oxides. Chem. Rev. 114, 10613–10653 (2014).
Sattler, J. J. H. B. et al. Operando UV-Vis spectroscopy of a catalytic solid in a pilot-scale reactor: deactivation of a CrOx/Al2O3 propane dehydrogenation catalyst. Chem. Comm. 49, 1518–1520 (2013).
Vu, B. K. et al. Electronic density enrichment of Pt catalysts by coke in the propane dehydrogenation. Korean J. Chem. Eng. 28, 383–387 (2011).
Lorberth, J. et al. Synthesis of gallane-amine adducts as potential precursors for GaAs and (AlGa) as MOVPE processes and the crystal structure of the {gallane·1,3-bis(dimethylamino)propane} adduct H3Ga·N(CH3)2(CH2)3N(CH3)2 . Adv. Mater. 4, 576–579 (1992).
Greenwood, N. N., Storr, A. & Wallbridge, M. G. H. Trimethylamine adducts of gallane and trideuteriogallane. Inorg. Chem. 2, 1036–1039 (1963).
Naidich, J. V. & Chuvashov, J. N. Wettability and contact interaction of gallium-containing melts with non-metallic solids. J. Mat. Sci. 18, 2071–2080 (1983).
Hardy, S. C. The surface tension of liquid gallium. J. Cryst. Growth 71, 602–606 (1985).
Doudrick, K. et al. Different shades of oxide: from nanoscale wetting mechanisms to contact printing of gallium-based liquid metals. Langmuir 30, 6867–6877 (2014).
Armbrüster, M., Schlögl, R. & Grin, Y. Intermetallic compounds in heterogeneous catalysis—a quickly developing field. Sci. Technol. Adv. Mater. 15, 034803 (2014).
Niedermaier, I., Kolbeck, C., Steinrück, H. P. & Maier, F. Dual analyzer system for surface analysis dedicated for angle-resolved photoelectron spectroscopy at liquid surfaces and interfaces. Rev. Sci. Instrum. 87, 045105 (2016).
Rodriguez, L. et al. Dehydrogenation of n-butane over Pd–Ga/Al2O3 catalysts. Appl. Cat. A 373, 66–70 (2010).
Penner, S. et al. Pd/Ga2O3 methanol steam reforming catalysts: Part I. Morphology, composition and structural aspects. Appl. Cat. A 358, 193–202 (2009).
Haghofer, A. et al. In situ study of the formation and stability of supported Pd2Ga methanol steam reforming catalysts. J. Catal. 286, 13–21 (2012).
Braunagel, N. A. & Melas, A. Gallium hydride/trialkylamine adducts, and their use in deposition of III–V compound films. European patent EP0251555A1 (1988).
Bove, L. E. et al. Vibrational dynamics of liquid gallium at 320 and 970 K. Phys. Rev. B 71, 014207 (2005).
Acknowledgements
The Cluster of Excellence-Engineering of Advanced Material is acknowledged for financial support.
Author information
Authors and Affiliations
Contributions
N.T, J.D. and P.W. conceived and designed the experiments. N.T. synthesized the catalytic materials and J.D. performed the catalytic testing. M.D. and W.P. contributed the electron microscopic analysis, M.B. and R.H. the XRD characterization and M.G., C.P., F.M., H.-P.S. the XPS characterization of the materials and the XPS measurements of the model system. J.E., C.N. and A.G. performed the molecular dynamic simulations. All the authors discussed the results and co-wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 1892 kb)
Rights and permissions
About this article
Cite this article
Taccardi, N., Grabau, M., Debuschewitz, J. et al. Gallium-rich Pd–Ga phases as supported liquid metal catalysts. Nature Chem 9, 862–867 (2017). https://doi.org/10.1038/nchem.2822
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.2822
This article is cited by
-
Liquid metals for boosting stability of zeolite catalysts in the conversion of methanol to hydrocarbons
Nature Communications (2024)
-
Dry reforming of methane over gallium-based supported catalytically active liquid metal solutions
Communications Chemistry (2023)
-
Achiral organoiodine-functionalized helical polyisocyanides for multiple asymmetric dearomative oxidations
Nature Communications (2023)
-
Low-temperature non-equilibrium synthesis of anisotropic multimetallic nanosurface alloys for electrochemical CO2 reduction
Nature Synthesis (2023)
-
Current state and future prospects of liquid metal catalysis
Nature Catalysis (2023)