Abstract
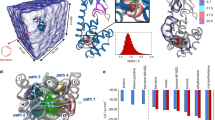
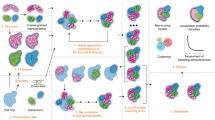
Protein–protein association is fundamental to many life processes. However, a microscopic model describing the structures and kinetics during association and dissociation is lacking on account of the long lifetimes of associated states, which have prevented efficient sampling by direct molecular dynamics (MD) simulations. Here we demonstrate protein–protein association and dissociation in atomistic resolution for the ribonuclease barnase and its inhibitor barstar by combining adaptive high-throughput MD simulations and hidden Markov modelling. The model reveals experimentally consistent intermediate structures, energetics and kinetics on timescales from microseconds to hours. A variety of flexibly attached intermediates and misbound states funnel down to a transition state and a native basin consisting of the loosely bound near-native state and the tightly bound crystallographic state. These results offer a deeper level of insight into macromolecular recognition and our approach opens the door for understanding and manipulating a wide range of macromolecular association processes.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Scott, D. E., Bayly, A. R., Abell, C. & Skidmore, J. Small molecules, big targets: drug discovery faces the protein–protein interaction challenge. Nat. Rev. Drug. Discov. 15, 533–550 (2016).
Doench, J. G. et al. Rational design of highly active sgrnas for crispr–cas9-mediated gene inactivation. Nat. Biotechnol. 32, 1262–1267 (2014).
King, N. P. et al. Computational design of self-assembling protein nanomaterials with atomic level accuracy. Science 336, 1171–1174 (2012).
Schreiber, G., Haran, G. & Zhou, H.-X. Fundamental aspects of protein–protein association kinetics. Chem. Rev. 109, 839–860 (2009).
Tang, C., Iwahara, J. & Clore, G. M. Visualization of transient encounter complexes in protein–protein association. Nature 444, 383–386 (2006).
Gabdoulline, R. R. & Wade, R. C. Simulation of the diffusional association of barnase and barstar. Biophys. J. 72, 1917–1929 (1997).
Spaar, A., Dammer, C., Gabdoulline, R. R., Wade, R. C. & Helms, V. Diffusional encounter of barnase and barstar. Biophys. J. 90, 1913–1924 (2006).
Levy, Y., Wolynes, P. G. & Onuchic, J. N. Protein topology determines binding mechanism. Proc. Natl Acad. Sci. USA 101, 511–516 (2004).
Schluttig, J., Alamanova, D., Helms, V. & Schwarz, U. S. Dynamics of protein–protein encounter: a langevin equation approach with reaction patches. J. Chem. Phys. 129, 155106 (2008).
Gumbart, J. C., Roux, B. & Chipot, C. Efficient determination of protein–protein standard binding free energies from first principles. J. Chem. Theory Comput. 9, 3789–3798 (2013).
Barducci, A., Bonomi, M., Prakash, M. K. & Parrinello, M. Free-energy landscape of protein oligomerization from atomistic simulations. Proc. Natl Acad. Sci. USA 110, E4708–E4713 (2013).
Tiwary, P. & Parrinello, M. From metadynamics to dynamics. Phys. Rev. Lett. 111, 230602 (2013).
Wu, H., Paul, F., Wehmeyer, C. & Noé, F. Multiensemble Markov models of molecular thermodynamics and kinetics. Proc. Natl Acad. Sci. USA 113, E3221–E3230 (2016).
Prinz, J.-H. et al. Markov models of molecular kinetics: generation and validation. J. Chem. Phys. 134, 174105 (2011).
Lindorff-Larsen, K., Piana, S., Dror, R. O. & Shaw, D. E. How fast-folding proteins fold. Science 334, 517–520 (2011).
Buch, I., Giorgino, T. & De Fabritiis, G. Complete reconstruction of an enzyme-inhibitor binding process by molecular dynamics simulations. Proc. Natl Acad. Sci. USA 108, 10184–10189 (2011).
Kohlhoff, K. J. et al. Cloud-based simulations on Google Exacycle reveal ligand modulation of GPCR activation pathways. Nat. Chem. 6, 15–21 (2014).
Plattner, N. & Noé, F. Protein conformational plasticity and complex ligand binding kinetics explored by atomistic simulations and Markov models. Nat. Commun. 6, 7653 (2015).
Silva, D.-A., Bowman, G. R., Sosa-Peinado, A. & Huang, X. A role for both conformational selection and induced fit in ligand binding by the Lao protein. PLoS Comput. Biol. 7, e1002054 (2011).
Piana, S., Lindorff-Larsen, K. & Shaw, D. E. Atomistic description of the folding of a dimeric protein. J. Phys. Chem. B 117, 12935–12942 (2013).
Ahmad, M., Gu, W., Geyer, T. & Helms, V. Adhesive water networks facilitate binding of protein interfaces. Nat. Commun. 2, 261 (2011).
Schreiber, G. & Fersht, A. R. Rapid, electrostatically assisted association of proteins. Nat. Struct. Biol. 3, 427–431 (1996).
Schreiber, G. & Fersht, A. R. Interaction of barnase with its polypeptide inhibitor barstar studied by protein engineering. Biochemistry 32, 5145–5150 (1993).
Hartley, R. W. Directed mutagenesis and barnase–barstar recognition. Biochemistry 32, 5978–5984 (1993).
Doerr, S. & Fabritiis, G. D. On-the-fly learning and sampling of ligand binding by high-throughput molecular simulations. J. Chem. Theory Comput. 10, 2064–2069 (2014).
Bowman, G. R., Ensign, D. L. & Pande, V. S. Enhanced modeling via network theory: adaptive sampling of Markov state models. J. Chem. Theory Comput. 6, 787–794 (2010).
Preto, J. & Clementi, C. Fast recovery of free energy landscapes via diffusion-map-directed molecular dynamics. Phys. Chem. Chem. Phys. 16, 19181–19191 (2014).
Bowman, G. R., Pande, V. S. & Noé, F. (eds.) An Introduction to Markov State Models and Their Application to Long Timescale Molecular Simulation (Vol. 797 of Advances in Experimental Medicine and Biology, Springer, 2014).
Sarich, M. & Schütte, C. Metastability and Markov State Models in Molecular Dynamics (Courant Lecture Notes, American Mathematical Society, 2013).
Noé, F., Wu, H., Prinz, J.-H. & Plattner, N. Projected and hidden Markov models for calculating kinetics and metastable states of complex molecules. J. Chem. Phys. 139, 184114 (2013).
Northrup, S. H., Allison, S. & McCammon, J. Brownian dynamics of diffusion-influenced bimolecular reactions. J. Chem. Phys. 80, 1517–1524 (1984).
Schreiber, G. & Fersht, A. R. Energetics of protein–protein interactions: analysis of the barnase–barstar interface by single mutations and double mutant cycles. J. Mol. Biol. 248, 478–486 (1995).
Matysiak, S. & Clementi, C. Optimal combination of theory and experiment for the characterization of the protein folding landscape of S6: how far can a minimalist model go? J. Mol. Biol. 343, 235–248 (2004).
Frisch, C., Fersht, A. R. & Schreiber, G. Experimental assignment of the structure of the transition state for the association of barnase and barstar. J. Mol. Biol. 308, 69–77 (2001).
Harel, M., Cohen, M. & Schreiber, G. On the dynamic nature of the transition state for protein–protein association as determined by double-mutant cycle analysis and simulation. J. Mol. Biol. 371, 180–196 (2007).
Chung, H. S., Louis, J. M. & Eaton, W. A. Single-molecule fluorescence experiments determine protein folding transition path times. Science 335, 981–984 (2012).
Anunciado, D., Dhar, A., Gruebele, M. & Baranger, A. M. Multistep kinetics of the U1A–SL2 RNA complex dissociation. J. Mol. Biol. 408, 896–908 (2011).
Buckle, A. M., Schreiber, G. & Fersht, A. R. Protein–protein recognition: crystal structural analysis of a barnase–barstar complex at 2.0-Å resolution. Biochemistry 33, 8878–8889 (1994).
Case, D. A. et al. The Amber biomolecular simulation programs. J. Comput. Chem. 26, 1668–1688 (2005).
Hornak, V. et al. Comparison of multiple Amber force fields and development of improved protein backbone parameters. Proteins 65, 712–725 (2006).
Jorgensen, W. L., Chandrasekhar, J., Madura, J. D., Impey, R. W. & Klein, M. L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 79, 926–935 (1983).
Harvey, M. J., Giupponi, G. & De Fabritiis, G. ACEMD: Accelerated molecular dynamics simulations in the microseconds timescale. J. Chem. Theory Comp. 5, 1632–1639 (2009).
Buch, I., Harvey, M. J., Giorgino, T., Anderson, D. P. & De Fabritiis, G. High-throughput all-atom molecular dynamics simulations using distributed computing. J. Chem. Inf. Model. 50, 397–403 (2010).
Best, R. B. et al. Optimization of the additive CHARMM all-atom protein force field targeting improved sampling of the backbone φ, ψ and side-chain χ1 and χ2 dihedral angles. J. Chem. Theo. Comp. 8, 3257–3273 (2012).
Doerr, S., Harvey, M. J., Noé, F. & Fabritiis, G. D. HTMD: high-throughput molecular dynamics for molecular discovery. J. Chem. Theory Comput. 12, 1845–1852 (2016).
Scherer, M. K. et al. PyEMMA 2: a software package for estimation, validation and analysis of Markov models. J. Chem. Theory Comput. 11, 5525–5542 (2015).
Perez-Hernandez, G., Paul, F., Giogino, T., De Fabritiis, G. & Noé, F. Identification of slow molecular order parameters for Markov model construction. J. Chem. Phys. 139, 015102 (2013).
Molgedey, L. & Schuster, H. G. Separation of a mixture of independent signals using time delayed correlations. Phys. Rev. Lett. 72, 3634–3637 (1994).
Trendelkamp-Schroer, B., Wu, H., Paul, F. & Noé, F. Estimation and uncertainty of reversible Markov models. J. Chem. Phys. 143, 174101 (2015).
Noé, F. & Nüske, F. A variational approach to modeling slow processes in stochastic dynamical systems. Multiscale Model. Simul. 11, 635–655 (2013).
Acknowledgements
We are grateful to the following researchers for inspiring discussions and extensive feedback on the manuscript: J. Clark, C. Clementi, O. Daumke, W. A. Eaton, M. Gruebele, K. Lindorff-Larsen, S. Olsson, F. Paul, B. Roux, U. Schwarz, S. Sukenik, R. Wade and T. Weikl. Funding is acknowledged from the European Commission (ERC StG pcCells and PRACE project 2014102337 to F.N.), Deutsche Forschungsgemeinschaft (NO 825/3-1, SFB 958/A4, SFB 740/D7 to F.N.), Einstein Foundation Berlin (project SoOPic to N.P.), MINECO (BIO2014-53095-P) and FEDER (to G.D.F.), Acellera Ltd. (to S.D.). We thank the volunteers of GPUGRID for donating computing time for simulations.
Author information
Authors and Affiliations
Contributions
N.P., S.D., G.D.F and F.N. designed research and developed/implemented software. N.P. and S.D. conducted simulations. N.P., S.D. and F.N. analysed simulations. F.N. developed methods. N.P. and F.N. wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 4823 kb)
Supplementary information
Supplementary Movie 1 (MP4 1533 kb)
Supplementary information
Supplementary Movie 2 (MP4 1352 kb)
Rights and permissions
About this article
Cite this article
Plattner, N., Doerr, S., De Fabritiis, G. et al. Complete protein–protein association kinetics in atomic detail revealed by molecular dynamics simulations and Markov modelling. Nature Chem 9, 1005–1011 (2017). https://doi.org/10.1038/nchem.2785
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.2785
This article is cited by
-
Machine learning coarse-grained potentials of protein thermodynamics
Nature Communications (2023)
-
Differences in ligand-induced protein dynamics extracted from an unsupervised deep learning approach correlate with protein–ligand binding affinities
Communications Biology (2022)
-
A litmus test for classifying recognition mechanisms of transiently binding proteins
Nature Communications (2022)
-
Dynamic stability of salt stable cowpea chlorotic mottle virus capsid protein dimers and pentamers of dimers
Scientific Reports (2022)
-
Classification of protein–protein association rates based on biophysical informatics
BMC Bioinformatics (2021)