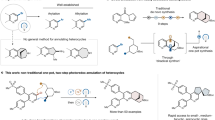
Abstract
Pentoses and hexoses contain more than three oxygen-bearing stereocentres and are ideal starting materials for the synthesis of multiply oxygenated natural products such as sagittamide D, maitotoxin and hikizimycin. Here we demonstrate new radical–radical homocoupling reactions of sugar derivatives with minimal perturbation of their chiral centres. The radical exchange procedure using Et3B/O2 converted sugar-derived α-alkoxyacyl tellurides into α-alkoxy radicals via decarbonylation and rapidly dimerized the monomeric radicals. The robustness of this process was demonstrated by a single-step preparation of 12 stereochemically diverse dimers with 6–10 secondary hydroxy groups, including the C5–C10 stereohexad of sagittamide D and the enantiomer of the C51–C60 stereodecad of maitotoxin. Furthermore, the optimally convergent radical–radical cross-coupling reaction achieved a one-step assembly of the protected C1–C11 oxygenated carbon chain of the anthelmintic hikizimycin. These exceptionally efficient homo- and heterocoupling methods together provide a powerful strategy for the expedited total synthesis of contiguously hydroxylated natural products.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Butler, M. S., Robertson, A. A. B. & Cooper, M. A. Natural product and natural product derived drugs in clinical trials. Nat. Prod. Rep. 31, 1612–1661 (2014).
Harvey, A. L., Edrada-Ebel, R. & Quinn, R. J. The re-emergence of natural products for drug discovery in the genomics era. Nat. Rev. Drug. Discov. 14, 111–129 (2015).
Rodrigues, T., Reker, D., Schneider, P. & Schneider, G. Counting on natural products for drug design. Nat. Chem. 8, 531–541 (2016).
Newman, D. J. & Cragg, G. M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 79, 629–661 (2016).
Lievens, S. C., Morinaka, B. I. & Molinski, T. F. Stereochemical elucidation of new sagittamides C–F from a didemnid ascidian. Aust. J. Chem. 63, 935–941 (2010).
Murata, M., Naoki, H., Matsunaga, S., Satake, M. & Yasumoto, T. Structure and partial stereochemical assignments for maitotoxin, the most toxic and largest natural non-biopolymer. J. Am. Chem. Soc. 116, 7098–7107 (1994).
Das, B. C., Defaye, J. & Uchida, K. The structure of hikizimycin. Part 1. Identification of 3-amino-3-deoxy-D-glucose and cytosine as structural components. Carbohydr. Res. 22, 293–299 (1972).
Uchida, K. & Das, B. C. Hikosamine, a novel C11 aminosugar component of the antibiotic hikizimycin. Biochimie 55, 635–636 (1973).
Vuilhorgne, M. et al. Carbon-13 nuclear magnetic resonance spectroscopy of naturally occurring substances. 54. Structure analysis of the nucleoside disaccharide antibiotic anthelmycin by carbon-13 nuclear magnetic resonance spectroscopy. A structural revision of hikizimycin and its identity with anthelmycin. J. Org. Chem. 42, 3289–3291 (1977).
Seike, H., Ghosh, I. & Kishi, Y. Stereochemistry of sagittamide A: prediction and confirmation. Org. Lett. 8, 3865–3868 (2006).
Nicolaou, K. C. et al. Chemical synthesis of the GHIJKLMNO ring system of maitotoxin. J. Am. Chem. Soc. 130, 7466–7476 (2008).
Secrist, J. A. & Barnes, K. D. Synthesis of methyl peracetyl α-hikosaminide, the undecose portion of the nucleoside antibiotic hikizimycin. J. Org. Chem. 45, 4526–4528 (1980).
Danishefsky, S. J. & Maring, C. J. A stereoselective totally synthetic route to methyl α-peracetylhikosaminide. J. Am. Chem. Soc. 111, 2193–2204 (1989).
Ikemoto, N. & Schreiber, S. L. Total synthesis of (–)-hikizimycin employing the strategy of two-directional chain synthesis. J. Am. Chem. Soc. 114, 2524–2536 (1992).
Fürstner, A. & Wuchrer, M. Concise approach to the ‘higher sugar’ core of the nucleoside antibiotic hikizimycin. Chem. Eur. J. 12, 76–89 (2006).
Casiraghi, G., Zanardi, F., Rassu, G. & Spanu, P. Stereoselective approaches to bioactive carbohydrates and alkaloids—with a focus on recent syntheses drawing from the chiral pool. Chem. Rev. 95, 1677–1716 (1995).
Nicolaou, K. C. & Sorensen, E. J. Classics in Total Synthesis: Targets, Strategies, Methods (Wiley-VCH, 1996).
Wilson, R. M. & Danishefsky, S. J. Pattern recognition in retrosynthetic analysis: snapshots in total synthesis. J. Org. Chem. 72, 4293–4305 (2007).
Hanessian, S., Giroux, S. & Merner, B. L. Design and Strategy in Organic Synthesis from the Chiron Approach to Catalysis (Wiley-VCH, 2013).
Northrup, A. B. & MacMillan, D. W. C . Two-step synthesis of carbohydrates by selective aldol reactions. Science 305, 1752–1755 (2004).
Aljahdali, A. Z., Shi, P., Zhong, Y. & O'Doherty, G. A. De novo asymmetric synthesis of the pyranoses: from monosaccharides to oligosaccharides. Adv. Carbohydr. Chem. Biochem. 69, 55–123 (2013).
Frihed, T. G., Bols, M. & Pedersen, C. M. Synthesis of L-hexoses. Chem. Rev. 115, 3615–3676 (2015).
Qin, T. et al. A general alkyl–alkyl cross-coupling enabled by redox-active esters and alkylzinc reagents. Science 352, 801–805 (2016).
Jana, R., Pathak, T. P. & Sigman, M. S. Advances in transition metal (Pd, Ni, Fe)-catalyzed cross-coupling reactions using alkyl-organometallics as reaction partners. Chem. Rev. 111, 1417–1492 (2011).
Weiper, A. & Schäfer, H.-J. Mixed Kolbe electrolyses with sugar carboxylic acids. Angew. Chem. Int. Ed. Engl. 29, 195–197 (1990).
Schäfer, H.-J. Recent contributions of Kolbe electrolysis to organic synthesis. Top. Curr. Chem. 152, 91–151 (1990).
Moeller, K. D. Synthetic applications of anodic electrochemistry. Tetrahedron 56, 9527–9554 (2000).
Giese, B., Rückert, B., Gröninger, K. S., Muhn, R. & Lindner, H. J. Dimerization of carbohydrate radicals. Liebigs Ann. Chem. 997–1000 (1988).
Doisneau, G. & Beau, J.-M. Radical dimerization of glycosyl 2-pyridylsulfones with samarium(II) iodide in the presence of HMPA. Tetrahedron Lett. 39, 3477–3480 (1998).
Guerrini, M., Guglieri, S., Santarsiero, R. & Vismara, E. Synthesis and characterisation of hexa- and tetrasaccharide mimics from acetobromomaltotriose and acetobromomaltose, and of C-disaccharide mimics from acetobromoglucose, obtained by electrochemical reduction on silver. Tetrahedron: Asymmetry 16, 243–253 (2005).
Namy, J. L., Souppe, J. & Kagan, H. B. Efficient formation of pinacols from aldehydes or ketones mediated by samarium diiodide. Tetrahedron Lett. 24, 765–766 (1983).
Barden, M. C. & Schwartz, J. Stereoselective ‘pinacol’ coupling of 2,3-O-isopropylidene-D-glyceraldehyde. J. Org. Chem. 62, 7520–7521 (1997).
Ollivier, C. & Renaud, P. Organoboranes as a source of radicals. Chem. Rev. 101, 3415–3434 (2001).
Nagatomo, M., Nishiyama, H., Fujino, H. & Inoue, M. Decarbonylative radical coupling of α-aminoacyl tellurides: single-step preparation of γ-amino and α,β-diamino acids and rapid synthesis of gabapentin and manzacidin A. Angew. Chem. Int. Ed. 54, 1537–1541 (2015).
Nagatomo, M., Kamimura, D., Matsui, Y., Masuda, K. & Inoue, M. Et3B-mediated two- and three-component coupling reactions via radical decarbonylation of α-alkoxyacyl tellurides: single-step construction of densely oxygenated carboskeletons. Chem. Sci. 6, 2765–2769 (2015).
Matsumura, S., Matsui, Y., Nagatomo, M. & Inoue, M. Stereoselective construction of anti- and syn-1,2-diol structures via decarbonylative radical coupling of α-alkoxyacyl tellurides. Tetrahedron 72, 4859–4866 (2016).
Tel'noi, V. I. & Sheiman, M. S. Thermodynamics of organoselenium and organotellurium compounds. Russ. Chem. Rev. 64, 309–316 (1995).
Boger, D. L. & Mathvink, R. J. Acyl radicals: intermolecular and intramolecular alkene addition reactions. J. Org. Chem. 57, 1429–1443 (1992).
Chatgilialoglu, C., Crich, D., Komatsu, M. & Ryu, I. Chemistry of acyl radicals. Chem. Rev. 99, 1991–2070 (1999).
De Mico, A., Margarita, R., Parlanti, L., Vescovi, A. & Piancatelli, G. A versatile and highly selective hypervalent iodine(III)/2,2,6,6-tetramethyl-1-piperidinyloxyl-mediated oxidation of alcohols to carbonyl compounds. J. Org. Chem. 62, 6974–6977 (1997).
Krohn, K. & Börner, G. From sugars to carbocycles. 2. Three- to seven-membered rings from mannose by addition of 1,3-dithiane followed by intramolecular displacement reaction. J. Org. Chem. 59, 6063–6068 (1994).
Dupuis, J. et al. Conformation of glycosyl radicals: radical stabilization by β-CO bonds. Angew. Chem. Int. Ed. Engl. 23, 896–898 (1984).
Giese, B. The stereoselectivity of intermolecular free radical reactions [new synthetic methods (78)]. Angew. Chem. Int. Ed. Engl. 28, 969–980 (1989).
Rychnovsky, S. D., Powers, J. P. & LePage, T. J. Conformation and reactivity of anomeric radicals. J. Am. Chem. Soc. 114, 8375–8384 (1992).
Beckwith, A. L. J. & Duggan, P. J. The quasi-homo-anomeric interaction in substituted tetrahydropyranyl radicals: diastereoselectivity. Tetrahedron 54, 6919–6928 (1998).
Togo, H., He, W., Waki, Y. & Yokoyama, M. C-Glycosidation technology with free radical reactions. Synlett 700–717 (1998).
Praly, J.-P. Structure of anomeric glycosyl radicals and their transformations under reductive conditions. Adv. Carbohydr. Chem. Biochem. 56, 65–151 (2000).
Abe, H., Shuto, S. & Matsuda, A. Highly α- and β-selective radical C-glycosylation reactions using a controlling anomeric effect based on the conformational restriction strategy. A study on the conformation−anomeric effect−stereoselectivity relationship in anomeric radical reactions. J. Am. Chem. Soc. 123, 11870–11882 (2001).
Urabe, D., Asaba, T. & Inoue, M. Convergent strategies in total syntheses of complex terpenoids. Chem. Rev. 115, 9207–9231 (2015).
Acknowledgements
This research was financially supported by the Funding Program for a Grant-in-Aid for Scientific Research (A) (JSPS Grant no. 26253003) to M.I., and a Grant-in-Aid for Scientific Research (C) (JSPS Grant no. 16K08156) to M.N. A Fellowship from JSPS to K.M. is gratefully acknowledged. We thank D. Kamimura (Kaken Pharmaceutical) for conducting the preliminary experiments for dimerization reactions. This paper is dedicated to Professor Samuel J. Danishefsky on the occasion of his 80th birthday.
Author information
Authors and Affiliations
Contributions
K.M., M.N. and M.I. conceived and designed the study. K.M. and M.N. performed the syntheses and M.N. and M.I. co-wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 13422 kb)
Rights and permissions
About this article
Cite this article
Masuda, K., Nagatomo, M. & Inoue, M. Direct assembly of multiply oxygenated carbon chains by decarbonylative radical–radical coupling reactions. Nature Chem 9, 207–212 (2017). https://doi.org/10.1038/nchem.2639
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.2639
This article is cited by
-
Stereoselective assembly of C-oligosaccharides via modular difunctionalization of glycals
Nature Communications (2024)
-
Dehydroxylative radical N-glycosylation of heterocycles with 1-hydroxycarbohydrates enabled by copper metallaphotoredox catalysis
Nature Communications (2024)
-
N-glycoside synthesis through combined copper- and photoredox-catalysed N-glycosylation of N-nucleophiles
Nature Synthesis (2024)
-
Visible light activation enables desulfonylative cross-coupling of glycosyl sulfones
Nature Synthesis (2022)
-
Exploiting photoredox catalysis for carbohydrate modification through C–H and C–C bond activation
Nature Reviews Chemistry (2022)