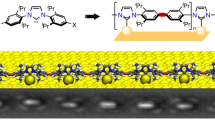
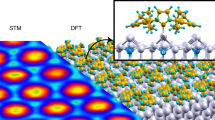
Abstract
Recently, N-heterocyclic carbenes (NHCs) were introduced as alternative anchors for surface modifications and so offered many attractive features, which might render them superior to thiol-based systems. However, little effort has been made to investigate the self-organization process of NHCs on surfaces, an important aspect for the formation of self-assembled monolayers (SAMs), which requires molecular mobility. Based on investigations with scanning tunnelling microscopy and first-principles calculations, we provide an understanding of the microscopic mechanism behind the high mobility observed for NHCs. These NHCs extract a gold atom from the surface, which leads to the formation of an NHC–gold adatom complex that displays a high surface mobility by a ballbot-type motion. Together with their high desorption barrier this enables the formation of ordered and strongly bound SAMs. In addition, this mechanism allows a complementary surface-assisted synthesis of dimeric and hitherto unknown trimeric NHC gold complexes on the surface.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Love, J. C., Estroff, L. A., Kriebel, J. K., Nuzzo, R. G. & Whitesides, G. M. Self-assembled monolayers of thiolates on metals as a form of nanotechnology. Chem. Rev. 105, 1103–1169 (2005).
Nuzzo, R. G. & Allara, D. L. Adsorption of bifunctional organic disulfides on gold surfaces. J. Am. Chem. Soc. 105, 4481–4483 (1983).
Veiseh, M., Wickes, B. T., Castner, D. G. & Zhang, M. Guided cell patterning on gold–silicon dioxide substrates by surface molecular engineering. Biomaterials 25, 3315–3324 (2004).
Hopkinson, M. N., Richter, C., Schedler, M. & Glorius, F. An overview of N-heterocyclic carbenes. Nature 510, 485–496 (2014).
Zhukhovitskiy, A. V., MacLeod, M. J. & Johnson, J. A. Carbene ligands in surface chemistry: from stabilization of discrete elemental allotropes to modification of nanoscale and bulk substrates. Chem. Rev. 115, 11503–11532 (2015).
Vougioukalakis, G. C. & Grubbs, R. H. Ruthenium-based heterocyclic carbene-coordinated olefin metathesis catalysts. Chem. Rev. 110, 1746–1787 (2010).
Fortman, G. C. & Nolan, S. P. N-Heterocyclic carbene (NHC) ligands and palladium in homogeneous cross-coupling catalysis: a perfect union. Chem. Soc. Rev. 40, 5151–5169 (2011).
Kantchev, E. A. B., O'Brien, C. J. & Organ, M. G. Palladium complexes of N-heterocyclic carbenes as catalysts for cross-coupling reactions—a synthetic chemist's perspective. Angew. Chem. Int. Ed. 46, 2768–2813 (2007).
Zhao, D., Candish, L., Paul, D. & Glorius, F. N-heterocyclic carbenes in asymmetric hydrogenation. ACS Catal. 6, 5978–5988 (2016).
Jacobsen, H., Correa, A., Poater, A., Costabile, C. & Cavallo, L. Understanding the M(NHC) (NHC = N-heterocyclic carbene) bond. Coord. Chem. Rev. 253, 687–703 (2009).
Marion, N., Ramon, R. S. & Nolan, S. P. [(NHC)Au(I)]-catalyzed acid-free alkyne hydration at part-per-million catalyst loadings. J. Am. Chem. Soc. 131, 448–449 (2009).
Weidner, T. et al. NHC-based self-assembled monolayers on solid gold substrates. Aust. J. Chem. 64, 1177–1179 (2011).
Zhukhovitskiy, A. V., Mavros, M. G., Van Voorhis, T. & Johnson, J. A. Addressable carbene anchors for gold surfaces. J. Am. Chem. Soc. 135, 7418–7421 (2013).
Crudden, C. M. et al. Ultra stable self-assembled monolayers of N-heterocyclic carbenes on gold. Nat. Chem. 6, 409–414 (2014).
Diaz Arado, O. et al. On-surface azide–alkyne cycloaddition on Au(111). ACS Nano 7, 8509–8515 (2013).
Zhong, D. et al. Linear alkane polymerization on a gold surface. Science 334, 213–216 (2011).
Griffiths, M. B. E. et al. Surfactant directed growth of gold metal nanoplates by chemical vapor deposition. Chem. Mater. 27, 6116–6124 (2015).
Johnson, J. A. & Zhukhovitskiy, A. V. Articles and methods comprising persistent carbenes and related compositions. WO Patent WO2014160471A2 (2014).
Voutchkova, A. M., Feliz, M., Clot, E., Eisenstein, O. & Crabtree, R. H. Imidazolium carboxylates as versatile and selective N-heterocyclic carbene transfer agents: synthesis, mechanism, and applications. J. Am. Chem. Soc. 129, 12834–12846 (2007).
Zhong, D., Wedeking, K., Chi, L., Erker, G. & Fuchs, H. Surface-mounted molecular rotors with variable functional groups and rotation radii. Nano Lett. 9, 4387–4391 (2009).
Barth, J. V., Brune, H., Ertl, G. & Behm, R. J. Scanning tunneling microscopy observations on the reconstructed Au(111) surface: atomic structure, long-range superstructure, rotational domains, and surface defects. Phys. Rev. B 42, 9307–9318 (1990).
Voigtländer, B., Meyer, G. & Amer, N. M. Epitaxial growth of thin magnetic cobalt films on Au(111) studied by scanning tunneling microscopy. Phys. Rev. B 44, 10354–10357 (1991).
Zhang, L. et al. Site- and configuration-selective anchoring of iron–phthalocyanine on the step edges of Au(111) surface. J. Phys. Chem. C 115, 10791–10796 (2011).
Bo, M., Morgenstern, K., Schneider, W.-D., Berndt, R. & Wo, C. Self-assembly of 1-nitronaphthalene on Au(111). Surf. Sci. 444, 199–210 (2000).
Xiao, W. D. et al. Impact of heterocirculene molecular symmetry upon two-dimensional crystallization. Sci. Rep. 4, 5415 (2014).
Zhang, H. et al. Surface supported gold–organic hybrids: on-surface synthesis and surface directed orientation. Small 10, 1361–1368 (2014).
Perera, U. G. et al. Controlled clockwise and anticlockwise rotational switching of a molecular motor. Nat. Nanotechnol. 8, 46–51 (2013).
Gimzewski, J. K. et al. Rotation of a single molecule within a supramolecular bearing. Science 281, 531–533 (1998).
Kumagai, M. & Ochiai, T. Development of a robot balancing on a ball. in Int. Conf. Control, Automation and Systems 433–438 (IEEE, 2008).
Maksymovych, P., Sorescu, D. C. & Yates, J. T. Jr. Gold-adatom-mediated bonding in self-assembled short-chain alkanethiolate species on the Au(111) surface. Phys. Rev. Lett. 97, 146103 (2006).
Gao, L. et al. Constructing an array of anchored single-molecule rotors on gold surfaces. Phys. Rev. Lett. 101, 197209 (2008).
Nguyen, H. C., Szyja, B. M. & Doltsinis, N. L. Electric conductance of a mechanically strained molecular junction from first principles: crucial role of structural relaxation and conformation sampling. Phys. Rev. B 90, 115440 (2014).
Weinberger, D. S. et al. Isolation of neutral mono- and dinuclear gold complexes of cyclic (alkyl)(amino)carbenes. Angew. Chem. Int. Ed. 52, 8964–8967 (2013).
Jerabek, P., Roesky, H. W., Bertrand, G. & Frenking, G. Coinage metals binding as main group elements: structure and bonding of the carbene complexes [TM(cAAC)2] and [TM(cAAC)2]+ (TM = Cu, Ag, Au). J. Am. Chem. Soc. 136, 17123–17135 (2014).
Crespo, J. et al. Ultrasmall NHC-coated gold nanoparticles obtained through solvent free thermolysis of organometallic Au(I) complexes. Dalton Trans 43, 15713–15718 (2014).
Acknowledgements
Financial support from the Deutsche Forschungsgemeinschaft (DFG) through the SFB 858 (projects B02 and B15), the Transregional Collaborative Research Center TRR 61 (projects B03 and B07), the Ministry of Science and Technology of China (no. 2013CBA01600), the National Natural Science Foundation of China (no. 61390501), the Leibniz award (F.G.) and the Fonds der Chemischen Industrie (J.B.E.) is gratefully acknowledged. We also thank O. Diaz-Arado and H. Mönig (both Westfälische Wilhelms-Universität) for support with the sample preparation for the XPS measurements.
Author information
Authors and Affiliations
Contributions
F.G. and H.F. initiated the project. F.G., H.F., G.W., A.R., S.A., N.D., M.K., J.B.E. and H.-Y.G. designed the experiments and coordinated the study. A.R., J.B.E. and C.R. synthesized the molecules. G.W. and M.K. performed the STM measurements. S.A. and N.D. performed DFT calculations. F.G., H.F., G.W., A.R., S.A., N.D., M.K., J.B.E., H.-J.G., H.-Y.G. and C.R. interpreted data. A.T. did the XPS experiments. H.F. and F.G. wrote the manuscript together with G.W., A.R., S.A. and N.D. All the authors read and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 2053 kb)
Supplementary information
Supplementary Movie 1 (MPG 4055 kb)
Rights and permissions
About this article
Cite this article
Wang, G., Rühling, A., Amirjalayer, S. et al. Ballbot-type motion of N-heterocyclic carbenes on gold surfaces. Nature Chem 9, 152–156 (2017). https://doi.org/10.1038/nchem.2622
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.2622
This article is cited by
-
Enhanced catalytic activity of N-heterocyclic carbene stabilized surface adatoms for CO reduction reaction
Communications Chemistry (2023)
-
On-surface synthesis of ballbot-type N-heterocyclic carbene polymers
Nature Chemistry (2023)
-
N-Heterocyclic carbene-based C-centered Au(I)-Ag(I) clusters with intense phosphorescence and organelle-selective translocation in cells
Nature Communications (2022)
-
Self-assembly of N-heterocyclic carbenes on Au(111)
Nature Communications (2021)
-
Controlled growth of ordered monolayers of N-heterocyclic carbenes on silicon
Nature Chemistry (2021)