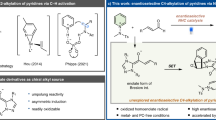
Abstract
Small-sized N-heterocycles are important structures in organic synthesis and medicinal chemistry. Palladium-catalysed intramolecular aminations of the C−H bonds of unfunctionalized amine precursors have recently emerged as an attractive new method for N-heterocycle synthesis. However, the way to control the reactivity of high-valent Pd intermediates to form the desired C−N cyclized products selectively remains poorly addressed. Herein we report a strategy to control the reductive elimination (RE) pathways in high-valent Pd catalysis and apply this strategy to achieve the synthesis of highly strained four-membered benzazetidines via the Pd-catalysed intramolecular C−H amination of N-benzyl picolinamides. These reactions represent the first practical synthetic method for benzazetidines and enable access to a range of complex benzazetidines from easily obtainable starting materials. The use of a newly designed phenyliodonium dimethylmalonate reagent is critical, as oxidation of Pd(II) palladacycles with this reagent favours a kinetically controlled C−N RE pathway to give strained ring-closed products.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Sweeney, J. B. Aziridines: epoxides’ ugly cousins? Chem. Soc. Rev. 31, 247–258 (2002).
Brandi, A., Cicchi, S. & Cordero, F. M. Novel syntheses of azetidines and azetidinones. Chem. Rev. 108, 3988–4035 (2008).
Alcaide, B., Almendros, P. & Aragoncillo, C. Highly reactive 4-membered ring nitrogen-containing heterocycles: synthesis and properties. Curr. Opin. Drug Discov. Devel. 13, 685–697 (2010).
Espino, C. G. & Du Bois, J. A Rh-catalyzed C−H insertion reaction for the oxidative conversion of carbamates to oxazolidinones. Angew. Chem. Int. Ed. 40, 598–600 (2001).
Hennessy, E. T. & Betley, T. A. Complex N-heterocycle synthesis via iron-catalyzed direct C–H bond amination. Science 340, 591–595 (2013).
Jeffrey, J. L. & Sarpong, R. Intramolecular C(sp3)–H amination. Chem. Sci. 4, 4092–4106 (2013).
Tsang, P. W. C., Zheng, N. & Buchwald, S. L. Combined C–H functionalization/C–N bond formation route to carbazoles. J. Am. Chem. Soc. 127, 14560–14561 (2005).
Wasa, M. & Yu, J.-Q. Synthesis of β, γ and δ-lactams via Pd(II)-catalyzed C–H activation reactions. J. Am. Chem. Soc. 130, 14058–14059 (2008).
McNally, A., Haffemayer, B., Collins, B. S. L. & Gaunt, M. J. Palladium-catalysed C–H activation of aliphatic amines to give strained nitrogen heterocycles. Nature 510, 129–133 (2014).
Chen, X., Engle, K. M., Wang, D.-H. & Yu, J.-Q. Palladium(II)-catalyzed C–H activation/C–C cross-coupling reactions: versatility and practicality. Angew. Chem. Int. Ed. 48, 5094–5115 (2009).
Lyons, T. W. & Sanford, M. S. Palladium-catalyzed ligand-directed C–H functionalization reactions. Chem. Rev. 110, 1147–1169 (2010).
Hickman, A. J. & Sanford, M. S. High-valent organometallic copper and palladium in catalysis. Nature 484, 177–185 (2012).
Engle, K. M., Mei, T.-S., Wang, X. & Yu, J.-Q. Bystanding F+ oxidants enable selective reductive elimination from high-valent metal centers in catalysis. Angew. Chem. Int. Ed. 50, 1478–1491 (2011).
Wojciechowski, K. Aza-ortho-xylylenes in organic synthesis. Eur. J. Org. Chem. 3587–3605 (2001).
Sadana, A. K., Saini, R. K. & Billups, W. E. Cyclobutarenes and related compounds. Chem. Rev. 1539–1602 (2003).
Burgess, E. M. & McCullagh, L. N-Phenylbenzoazetine. J. Am. Chem. Soc. 88, 1580–1581 (1966).
Beak, P. & Selling, G. W. Displacements at the nitrogen of lithio-alkoxylamides by organometallic reagents. J. Org. Chem. 54, 5574–5580 (1989).
Lancaster, M. & Smith, D. J. H. Preparation and some reactions of benzazetidines. Chem. Commun. 471–472 (1980).
Nadres, E. T. & Daugulis, O. Heterocycle synthesis via direct C−H/N−H coupling. J. Am. Chem. Soc. 134, 7–10 (2012).
He, G., Zhao, Y., Zhang, S., Lu, C. & Chen, G. Highly efficient syntheses of azetidines, pyrrolidines, and indolines via palladium-catalyzed intramolecular amination of C(sp3)–H and C(sp2)–H bonds at the γ and δ positions. J. Am. Chem. Soc. 134, 3–6 (2012).
He, G., Zhang, S., Nack, W. A. & Chen, G. Use of a readily removable auxiliary group for the synthesis of pyrrolidones by the palladium-catalyzed intramolecular amination of unactivated γ C(sp3)−H bonds. Angew. Chem. Int. Ed. 52, 11124–11128 (2013).
Engle, K. M. & Yu., J.-Q. Developing ligands for palladium(II)-catalyzed C–H functionalization. J. Org. Chem. 78, 8927–8955 (2013).
Gou, F.-R. et al. Palladium-catalyzed aryl C–H bonds activation/acetoxylation utilizing a bidentate system. Org. Lett. 11, 5726–5729 (2009).
Gary, J. B. & Sanford, M. S. Participation of carbonyl oxygen in carbon–carboxylate bond-forming reductive elimination from palladium. Organometallics 30, 6143–6149 (2011).
Nielsen, M. C., Lyngvi, E. & Schoenebeck, F. Chemoselectivity in the reductive elimination from high oxidation state palladium complexes—scrambling mechanism uncovered. J. Am. Chem. Soc. 135, 1978–1985 (2013).
Zhdankin, V. V. & Stang, P. J. Chemistry of polyvalent iodine. Chem. Rev. 108, 5299–5358 (2008).
Shabashov, M. & Daugulis, O. Auxiliary-assisted palladium-catalyzed arylation and alkylation of sp2 and sp3 carbon–hydrogen bonds. J. Am. Chem. Soc. 132, 3965–3972 (2010).
Deprez, N. R. & Sanford, M. S. Synthetic and mechanistic studies of Pd-catalyzed C−H arylation with diaryliodonium salts: evidence for a bi-metallic high oxidation state Pd intermediate. J. Am. Chem. Soc. 131, 11234–11241 (2009).
Powers, D. C., Benitez, D., Tkatchouk, E., Goddard, W. A. III & Ritter, T. Bimetallic reductive elimination from dinuclear Pd(III) complexes. J. Am. Chem. Soc. 132, 14092–14103 (2010).
Powers, D. C. et al. Connecting binuclear Pd(III) and mononuclear Pd(IV) chemistry by Pd−Pd bond cleavage. J. Am. Chem. Soc. 134, 12002–12009 (2012).
Acknowledgements
G.C. thanks the State Key Laboratory of Elemento-Organic Chemistry at Nankai University and the Pennsylvania State University for financial support for the experimental part of this work. P.L. thanks the University of Pittsburgh for financial support for the computational part of the work. Calculations were performed at the Center for Simulation and Modeling at the University of Pittsburgh and the Extreme Science and Engineering Discovery Environment (XSEDE) supported by the National Science Foundation.
Author information
Authors and Affiliations
Contributions
G.H. discovered the benzazetidine synthesis via the Pd-catalysed IDCA reaction of benzylamines, introduced the PhI(DMM) reagent, carried out most of the reaction optimization and structural determination of the reaction products and prepared the Supplementary Information. Z.G. helped in the preparation of some benzylamine substrates and contributed to the reaction optimization. G.L. conducted the computations. P.L. directed the computational studies. P.L. and G.L. prepared the computational sections of the manuscript. G.C. formulated the initial ideas of this work, supervised all the experiments, coordinated with P.L. on the computational studies and prepared most of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 20978 kb)
Supplementary information
Crystallographic data for compound 5. (CIF 13 kb)
Supplementary information
Structure factors file for compound 5. (FCF 157 kb)
Supplementary information
Crystallographic data for complexA. (CIF 16 kb)
Supplementary information
Structure factors file for complexA. (FCF 179 kb)
Supplementary information
Crystallographic data for compound 21N. (CIF 15 kb)
Supplementary information
Structure factors file for compound 21N. (FCF 178 kb)
Supplementary information
Crystallographic data for compound 26. (CIF 11 kb)
Supplementary information
Structure factors file for compound 26. (FCF 62 kb)
Supplementary information
Crystallographic data for palladacycle complexE. (CIF 25 kb)
Supplementary information
Structure factors file for palladacycle complexE. (FCF 351 kb)
Rights and permissions
About this article
Cite this article
He, G., Lu, G., Guo, Z. et al. Benzazetidine synthesis via palladium-catalysed intramolecular C−H amination. Nature Chem 8, 1131–1136 (2016). https://doi.org/10.1038/nchem.2585
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.2585
This article is cited by
-
Transition metal-catalysed directed C–H functionalization with nucleophiles
Nature Synthesis (2022)
-
Synthesis and characterization of new secondary benzylamines derivatives of aryl-himachalene
Chemical Papers (2021)
-
Electrooxidative para-selective C–H/N–H cross-coupling with hydrogen evolution to synthesize triarylamine derivatives
Nature Communications (2019)
-
Controlling Pd(iv) reductive elimination pathways enables Pd(ii)-catalysed enantioselective C(sp3)−H fluorination
Nature Chemistry (2018)