Abstract
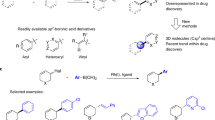
cross-coupling reactions between arylboronic acid and aryl halides are widely used in both academia and industry and are strategically important in the development of new agrochemicals and pharmaceuticals. cross-coupling reactions have been developed, but enantioselective variations are rare and simply retaining the stereochemistry is a problem. Here we report a highly enantioselective bond-forming method that couples arylboronic acids to racemic allyl chlorides. Both enantiomers of a cyclic chloride are converted into a single enantiomer of product via a dynamic kinetic asymmetric transformation. This Rh-catalysed method uses readily available and inexpensive building blocks and is mild and broadly applicable. For electron-deficient, electron-rich or ortho-substituted boronic acids better results are obtained with racemic allyl bromides. Oxygen substitution in the allyl halide is tolerated and the products can be functionalized to provide diverse building blocks. The approach fills a significant gap in the methods for catalytic asymmetric synthesis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
de Meijere, A., Brase, S. & Oestreich, M. Metal Catalyzed Cross-Coupling Reactions and More (Wiley, 2014).
Cooper, T. W. J., Campbell, I. B. & MacDonald, S. J. F. Factors determining the selection of organic reactions by medicinal chemists and the use of these reactions in arrays (small focused libraries). Angew. Chem. Int. Ed. 49, 8082–8091 (2010).
Nadin, A., Hattotuwagama, C. & Churcher, I. Lead-oriented synthesis: a new opportunity for synthetic chemistry. Angew. Chem. Int. Ed. 51, 1114–1122 (2012).
Roughley, S. D. & Jordan, A. M. The medicinal chemist's toolbox: an analysis of reactions used in the pursuit of drug candidates. J. Med. Chem. 54, 3451–3479 (2011).
Lennox, A. J. J. & Lloyd-Jones, G. C. Selection of boron reagents for Suzuki–Miyaura coupling. Chem. Soc. Rev. 43, 412–443 (2014).
Hall, D. G. Boronic Acids: Preparation and Applications in Organic Synthesis and Medicine (Wiley, 2006).
Howell, G. P. Asymmetric and diastereoselective conjugate addition reactions: C–C bond formation at large scale. Org. Process Res. Dev. 16, 1258–1272 (2012).
Jacobsen, E. N., Pfaltz, A. & Yamamoto, H. Comprehensive Asymmetric Catalysis: Supplement 2 (Springer, 2004).
Takaya, Y., Ogasawara, M. & Hayashi, T. Rhodium-catalyzed asymmetric 1,4-addition of aryl- and alkenylboronic acids to enones. J. Am. Chem. Soc. 3, 5579–5580 (1998).
Hayashi, T. & Yamasaki, K. Rhodium-catalyzed asymmetric 1,4-addition and its related asymmetric reactions. Chem. Rev. 103, 2829–2844 (2003).
Evans, P. A. Modern Rhodium-Catalyzed Organic Reactions (Wiley, 2005).
Tsuji, J., Minami, I. & Shimizu, I. Allyation of carbonucleophiles with allylic carbonates under neutral conditions catalyzed by rhodium complexes. Tetrahedron Lett. 25, 5157–5160 (1984).
Evans, P. A. & Kennedy, L. J. Enantiospecific and regioselective rhodium-catalyzed allylic alkylation: diastereoselective approach to quaternary carbon stereogenic centers. Org. Lett. 2, 2213–2215 (2000).
Evans, P. A. & Leahy, D. K. Regioselective and enantiospecific rhodium-catalyzed allylic alkylation reactions using copper(I) enolates: synthesis of (–)-sugiresinol dimethyl ether. J. Am. Chem. Soc. 125, 8974–8975 (2003).
Evans, P. A. & Uraguchi, D. Regio- and enantiospecific rhodium-catalyzed arylation of unsymmetrical fluorinated acyclic allylic carbonates: inversion of absolute configuration. J. Am. Chem. Soc. 125, 7158–7159 (2003).
Hayashi, T., Okada, A., Suzuka, T. & Kawatsura, M. High enantioselectivity in rhodium-catalyzed allylic alkylation of 1-substituted 2-propenyl acetates. Org. Lett. 5, 1713–1715 (2003).
Menard, F., Chapman, T. M., Dockendorff, C. & Lautens, M. Rhodium-catalyzed asymmetric allylic substitution with boronic acid nucleophiles. Org. Lett. 8, 4569–4572 (2006).
Menard, F., Perez, D., Sustac Roman, D., Chapman, T. M. & Lautens, M. Ligand-controlled selectivity in the desymmetrization of meso cyclopenten-1,4-diols via rhodium(I)-catalyzed addition of arylboronic acids. J. Org. Chem. 75, 4056–4068 (2010).
Lautens, M., Fagnou, K. & Hiebert, S. Transition metal-catalyzed enantioselective ring-opening reactions of oxabicyclic alkenes. Acc. Chem. Res. 36, 48–58 (2003).
Trost, B. M. & Van Vranken, D. L. Asymmetric transition metal-catalyzed allylic alkylations. Chem. Rev. 96, 395–422 (1996).
Lu, Z. & Ma, S. Metal-catalyzed enantioselective allylation in asymmetric synthesis. Angew. Chem. Int. Ed. 47, 258–297 (2008).
Huerta, F. F., Minidis, A. B. E. & Bäckvall, J. Racemisation in asymmetric synthesis. Dynamic kinetic resolution and related processes in enzyme and metal catalysis. Chem. Soc. Rev. 30, 321–331 (2001).
Vedejs, E. & Jure, M. Efficiency in nonenzymatic kinetic resolution. Angew. Chem. Int. Ed. 44, 3974–4001 (2005).
Trost, B. M. & Fandrick, D. R. Palladium-catalyzed dynamic kinetic asymmetric allylic alkylation with the DPPBA ligands. Aldrichim. Acta 40, 59–72 (2007).
Trost, B. M. & Thaisrivongs, D. A. Strategy for employing unstabilized nucleophiles in palladium-catalyzed asymmetric allylic alkylations. J. Am. Chem. Soc. 130, 14092–14093 (2008).
Misale, A., Niyomchon, S., Luparia, M. & Maulide, N. Asymmetric palladium-catalyzed allylic alkylation using dialkylzinc reagents: a remarkable ligand effect. Angew. Chem. Int. Ed. 53, 7068–7073 (2014).
Langlois, J.-B., Emery, D., Mareda, J. & Alexakis, A. Mechanistic identification and improvement of a direct enantioconvergent transformation in copper-catalyzed asymmetric allylic alkylation. Chem. Sci. 3, 1062–1069 (2012).
Ito, H., Kunii, S. & Sawamura, M. Direct enantio-convergent transformation of racemic substrates without racemization or symmetrization. Nature Chem. 2, 972–976 (2010).
You, H., Rideau, E., Sidera, M. & Fletcher, S. P. Non-stabilized nucleophiles in Cu-catalysed dynamic kinetic asymmetric allylic alkylation. Nature 517, 351–355 (2015).
Sidera, M. & Fletcher, S. P. Cu-catalyzed asymmetric addition of sp2-hybridized zirconium nucleophiles to racemic allyl bromides. Chem. Commun. 51, 5044–5047 (2015).
Hamilton, J. Y., Sarlah, D. & Carreira, E. M. Iridium-catalyzed enantioselective allylic vinylation. J. Am. Chem. Soc. 135, 994–997 (2013).
Yin, J. & Buchwald, S. L. A catalytic asymmetric Suzuki coupling for the synthesis of axially chiral biaryl compounds. J. Am. Chem. Soc. 122, 12051–12052 (2000).
Baudoin, O. The asymmetric Suzuki coupling route to axially chiral biaryls. Eur. J. Org. Chem. 4223–4229 (2005).
Lovering, F., Bikker, J. & Humblet, C. Escape from flatland: increasing saturation as an approach to improving clinical success. J. Med. Chem. 52, 6752–6756 (2009).
Clemons, P. A. et al. Small molecules of different origins have distinct distributions of structural complexity that correlate with protein-binding profiles. Proc. Natl Acad. Sci. USA 107, 18787–18792 (2010).
Sakai, M., Hayashi, H. & Miyaura, N. Rhodium-catalyzed conjugate addition of aryl- or 1-alkenylboronic acids to enones. Organometallics 16, 4229–4231 (1997).
Hayashi, T., Takahashi, M., Takaya, Y. & Ogasawara, M. Catalytic cycle of rhodium-catalyzed asymmetric 1, 4-addition of organoboronic acids. arylrhodium, oxa-π-allylrhodium, and hydroxorhodium intermediates. J. Am. Chem. Soc. 124, 5052–5058 (2002).
Puchot, C. et al. Nonlinear effects in asymmetric synthesis. examples in asymmetric oxidations and aldolization reactions. J. Am. Chem. Soc. 108, 2353–2357 (1986).
Acknowledgements
We acknowledge financial support from the Engineering and Physical Sciences Research Council (EP/H003711/1, a Career Acceleration Fellowship to S.F.).
Author information
Authors and Affiliations
Contributions
M.S. performed the experiments and S.F. guided the research. Both authors contributed to designing the experiments, analysing the data and writing the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors are named as inventors on a UK patent application filed by Isis Innovation, which is the technology transfer arm of the University of Oxford.
Supplementary information
Supplementary information
Supplementary information (PDF 5920 kb)
Rights and permissions
About this article
Cite this article
Sidera, M., Fletcher, S. Rhodium-catalysed asymmetric allylic arylation of racemic halides with arylboronic acids. Nature Chem 7, 935–939 (2015). https://doi.org/10.1038/nchem.2360
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.2360
This article is cited by
-
Chelation enables selectivity control in enantioconvergent Suzuki–Miyaura cross-couplings on acyclic allylic systems
Nature Chemistry (2024)
-
Migratory allylic arylation of 1,n-enols enabled by nickel catalysis
Nature Communications (2023)
-
Mechanistic investigation of Rh(i)-catalysed asymmetric Suzuki–Miyaura coupling with racemic allyl halides
Nature Catalysis (2021)
-
A catalytic asymmetric cross-coupling approach to the synthesis of cyclobutanes
Nature Chemistry (2021)
-
Highly enantioselective rhodium-catalyzed cross-coupling of boronic acids and racemic allyl halides
Nature Protocols (2019)