Abstract
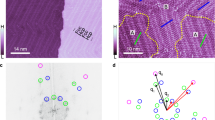
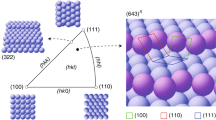
The homochirality of biomolecules is a signature of life on Earth and has significant implications in, for example, the production of pharmaceutical compounds. It has been suggested that biomolecular homochirality may have arisen from the amplification of a spontaneously formed small enantiomeric excess (e.e.). Many minerals exhibit naturally chiral surfaces and so adsorption has been proposed as one possible mechanism for such an amplification of e.e. Here we show that when gas-phase mixtures of D- and L-aspartic acid are exposed to an achiral Cu(111) surface, a small e.e. in the gas phase, e.e.g, leads to an amplification of the e.e. on the surface, e.e.s, under equilibrium conditions. Adsorption-induced amplification of e.e. does not require a chiral surface. The dependence of e.e.s on e.e.g has been modelled successfully using a Langmuir-like adsorption isotherm that incorporates the formation of homochiral adsorbate clusters on the surface.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Bonner, W. A. The origin and amplification of biomolecular chirality. Origins Life Evol. B. 21, 59–111 (1991).
Bada, J. L. Biomolecules—origins of homochirality. Nature 374, 594–595 (1995).
Weissbuch, I., Leiserowitz, L. & Lahav, M. Stochastic ‘mirror symmetry breaking’ via self-assembly, reactivity and amplification of chirality: relevance to abiotic conditions. Top. Curr. Chem. 259, 123–165 (2005).
Soai, K., Shibata, T., Morioka, H. & Choji, K. Asymmetric autocatalysis and amplification of enantiomeric excess of a chiral molecule. Nature 378, 767–768 (1995).
Soai, K. et al. D- and L-quartz-promoted highly enantioselective synthesis of a chiral organic compound. J. Am. Chem. Soc. 121, 11235–11236 (1999).
Green, M. M. et al. Majority rules in the co-polymerization of mirror image isomers. J. Am. Chem. Soc. 117, 4181–4182 (1995).
Viedma, C. Chiral symmetry breaking during crystallization: complete chiral purity induced by nonlinear autocatalysis and recycling. Phys. Rev. Lett. 94, 065504 (2005).
Klussmann, M. et al. Thermodynamic control of asymmetric amplification in amino acid catalysis. Nature 441, 621–623 (2006).
Perry, R. H., Chunping, W., Nefliu, M. & Cooks, R. G. Serine sublimes with spontaneous chiral amplification. Chem. Commun. 1071–1073 (2007).
Blackmond, D. G. & Klussmann, M. Spoilt for choice: assessing phase behavior models for the evolution of homochirality. Chem. Commun. 3990–3996 (2007).
Mallat, T., Orglmeister, E. & Baiker, A. Asymmetric catalysis at chiral metal surfaces. Chem. Rev. 107, 4863–4890 (2007).
Gellman, A. J. et al. Superenantioselective chiral surface explosions. J. Am. Chem. Soc. 135, 19208–19214 (2013).
Mastai, Y. Enantioselective crystallization on nanochiral surfaces. Chem. Soc. Rev. 38, 772–780 (2009).
Yun, Y. & Gellman, A. J. Enantioselective separation on naturally chiral metal surfaces: D,L-aspartic acid on Cu(3,1,17)R&S surfaces. Angew. Chem. Int. Ed. 52, 3394–3397 (2013).
Hazen, R. M., Filley, T. R. & Goodfriend, G. A. Selective adsorption of L- and D-amino acids on calcite: implications for biochemical homochirality. Proc. Natl Acad. Sci. USA 98, 5487–5490 (2001).
Hazen, R. M. & Sholl, D. S. Chiral selection on inorganic crystalline surfaces. Nature Mater. 2, 367–374 (2003).
Zaera, F. Chiral modification of solid surfaces: a molecular view. J. Phys. Chem. C 112, 16196–16203 (2008).
Gellman, A. J. Chiral surfaces: accomplishments and challenges. ACS Nano 4, 5–10 (2010).
McFadden, C. F., Cremer, P. S. & Gellman, A. J. Adsorption of chiral alcohols on “chiral” metal surfaces. Langmuir 12, 2483–2487 (1996).
Horvath, J. D., Baker, L. & Gellman, A. J. Enantiospecific orientation of R-3-methylcyclohexanone on the chiral Cu{643}R&S surfaces. J. Phys. Chem. C 112, 7637–7643 (2008).
Gladys, M. J. et al. Enantiospecific adsorption of alanine on the chiral Cu{531} surface. J. Phys. Chem. C 111, 8331–8336 (2007).
Rampulla, D. M., Francis, A. J., Knight, K. S. & Gellman, A. J. Enantioselective surface chemistry of R-2-bromobutane on Cu(643)R&S and Cu(531)R&S. J. Phys. Chem. B 110, 10411–10420 (2006).
Horvath, J. D. & Gellman, A. J. Enantiospecific desorption of R- and S-propylene oxide from a chiral Cu(643) surface. J. Am. Chem. Soc. 123, 7953–7954 (2001).
Horvath, J. D. & Gellman, A. J. Enantiospecific desorption of chiral compounds from chiral Cu(643) and achiral Cu(111) surfaces. J. Am. Chem. Soc. 124, 2384–2392 (2002).
Huang, Y. & Gellman, A. J. Enantiospecific adsorption of (R)-3-methylcyclohexanone on naturally chiral surfaces vicinal to Cu(110). Top. Catal. 54, 1403–1413 (2011).
Cheong, W. Y. & Gellman, A. J. Energetics of chiral imprinting of Cu(100) by lysine. J. Phys. Chem. C 115, 1031–1035 (2011).
Lorenzo, M. O., Baddeley, C. J., Muryn, C. & Raval, R. Extended surface chirality from supramolecular assemblies of adsorbed chiral molecules. Nature 404, 376–379 (2000).
Ernst, K. H. Supramolecular chirality. Top. Curr. Chem. 265, 209–252 (2006).
Barth, J. V. et al. Stereochemical effects in supramolecular self-assembly at surfaces: 1D versus 2D enantiomorphic ordering for PVBA and PEBA on Ag(111). J. Am. Chem. Soc. 124, 7991–8000 (2002).
Barlow, S. M. & Raval, R. Complex organic molecules at metal surfaces: bonding, organisation and chirality. Surf. Sci. Rep. 50, 201–341 (2003).
Parschau, M., Romer, S. & Ernst, K. H. Induction of homochirality in achiral enantiomorphous monolayers. J. Am. Chem. Soc. 126, 15398–15399 (2004).
Haq, S., Liu, N., Humblot, V., Jansen, A. P. J. & Raval, R. Drastic symmetry breaking in supramolecular organization of enantiomerically unbalanced monolayers at surfaces. Nature Chem. 1, 409–414 (2009).
Nanita, S. C., Takats, Z., Cooks, R. G., Myung, S. & Clemmer, D. E. Chiral enrichment of serine via formation, dissociation, and soft-landing of octameric cluster ions. J. Am. Soc. Mass Spectrom. 15, 1360–1365 (2004).
Nanita, S. C. & Cooks, R. G. Serine octamers: cluster formation, reactions, and implications for biomolecule homochirality. Angew. Chem. Int. Ed. 45, 554–569 (2006).
Cundy, K. C. & Crooks, P. A. Unexpected phenomenon in the high performance liquid-chromatographic analysis of racemic 14C-labeled nicotine—separation of enantiomers in a totally achiral system. J. Chromatogr. 281, 17–33 (1983).
Soloshonok, V. A. Remarkable amplification of the self-disproportionation of enantiomers on achiral-phase chromatography columns. Angew. Chem. Int. Ed. 45, 766–769 (2006).
Soloshonok, V. A., Roussel, C., Kitagawa, O. & Sorochinsky, A. E. Self-disproportionation of enantiomers via achiral chromatography: a warning and an extra dimension in optical purifications. Chem. Soc. Rev. 41, 4180–4188 (2012).
Fletcher, S. P., Jagt, R. B. C. & Feringa, B. An astrophysically-relevant mechanism for amino acid enantiomer enrichment. Chem. Commun. 2578–2580 (2007).
Mhatre, B. S. Super-Enantiospecific Autocatalytic Decomposition of Tartaric Acid and Aspartic Acid on Cu Surfaces PhD thesis, Carnegie Mellon Univ. (2013).
Yun, Y., Wei, D., Sholl, D. S. & Gellman, A. J. Equilibrium adsorption of D- and L-alanine mixtures on naturally chiral Cu{3,1,17}R&S surfaces. J. Phys. Chem. C 118, 14957–14966 (2014).
Roth, C., Parschau, M. & Ernst, K. H. Chiral reconstruction of a metal surface by adsorption of racemic malic acid. ChemPhysChem 12, 1572–1577 (2011).
Lawton, T. J. et al. Long range chiral imprinting of Cu(110) by tartaric acid. J. Phys. Chem. C 117, 22290–22297 (2013).
Chen, Q. & Richardson, N. V. Surface facetting induced by adsorbates. Progr. Surf. Sci. 73, 59–77 (2003).
Mhatre, B. S. et al. A window on surface explosions: tartaric acid on Cu(110). J. Phys. Chem. C 117, 7577–7588 (2013).
Zhao, X. Y., Zhao, R. G. & Yang, W. S. Scanning tunneling microscopy investigation of L-lysine adsorbed on Cu(001). Langmuir 16, 9812–9818 (2000).
Kuhnle, A., Linderoth, T. R., Hammer, B. & Besenbacher, F. Chiral recognition in dimerization of adsorbed cysteine observed by scanning tunnelling microscopy. Nature 415, 891–893 (2002).
Barlow, S. M. et al. Supramolecular assembly of strongly chemisorbed size- and shape-defined chiral clusters: S- and R-alanine on Cu(110). Langmuir 20, 7171–7176 (2004).
Schiffrin, A. et al. Self-assembly of L-methionine on Cu(111): steering chiral organization by substrate reactivity and thermal activation. J. Phys. Chem. C 113, 12101–12108 (2009).
Humblot, V. et al. Characterization of two-dimensional chiral self-assemblies L- and D-methionine on Au(111). Langmuir 30, 203–212 (2014).
Mahapatra, M. et al. Formation of chiral self-assembled structures of amino acids on transition-metal surfaces: alanine on Pd(111). J. Phys. Chem. C 118, 6856–6865 (2014).
Zhao, X. Y., Yan, H., Zhao, R. G. & Yang, W. S. Self-assembled structures of glycine on Cu(111). Langmuir 19, 809–813 (2003).
Yitamben, E. N. et al. Tracking amino acids in chiral quantum corrals. J. Phys. Chem. C 117, 11757–11763 (2013).
Acknowledgements
This work was funded by the US Department of Energy through Grant No. DE-FG02-12ER16330. A.J.G. acknowledges the hospitality of the Fritz-Haber Institute during the writing of this manuscript.
Author information
Authors and Affiliations
Contributions
Y.Y. made the initial observation of auto-amplification on a Cu{653} surface. A.J.G. and Y.Y. designed the experiments that demonstrated auto-amplification on the achiral Cu(111) surface. These were performed solely by Y.Y. A.J.G. and Y.Y. analysed the results and prepared the manuscript jointly.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Yun, Y., Gellman, A. Adsorption-induced auto-amplification of enantiomeric excess on an achiral surface. Nature Chem 7, 520–525 (2015). https://doi.org/10.1038/nchem.2250
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.2250
This article is cited by
-
Chirality control of a single carbene molecule by tip-induced van der Waals interactions
Nature Communications (2023)
-
Condensation and asymmetric amplification of chirality in achiral molecules adsorbed on an achiral surface
Nature Communications (2023)
-
Simultaneous switching of supramolecular chirality and organizational chirality driven by Coulomb expansion
Nano Research (2022)
-
Homochirality in biomineral suprastructures induced by assembly of single-enantiomer amino acids from a nonracemic mixture
Nature Communications (2019)
-
Single-molecule insights into surface-mediated homochirality in hierarchical peptide assembly
Nature Communications (2018)