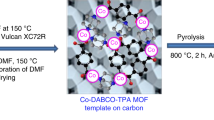
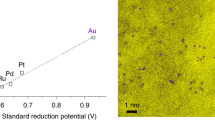
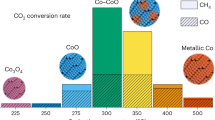
Abstract
Molecularly well-defined homogeneous catalysts are known for a wide variety of chemical transformations. The effect of small changes in molecular structure can be studied in detail and used to optimize many processes. However, many industrial processes require heterogeneous catalysts because of their stability, ease of separation and recyclability, but these are more difficult to control on a molecular level. Here, we describe the conversion of homogeneous cobalt complexes into heterogeneous cobalt oxide catalysts via immobilization and pyrolysis on activated carbon. The catalysts thus produced are useful for the industrially important reduction of nitroarenes to anilines. The ligand indirectly controls the selectivity and activity of the recyclable catalyst and catalyst optimization can be performed at the level of the solution-phase precursor before conversion into the active heterogeneous catalyst.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Vogt, P. F. & Gerulis, J. J. in Ullmann's Encyclopedia of Industrial Chemistry. http://dx.doi.org/10.1002/14356007.a02_037 (Wiley-VCH, 2000).
Ertl, G., Knözinger H. & Weitkamp, J. Handbook of Heterogeneous Catalysis (Wiley-VCH, 1997).
Baerns M. Basic Principles in Applied Catalysis (Springer, 2004).
Jones, A. C. & Hitchman, M. L. Chemical Vapour Deposition: Precursors, Processes and Applications 1–36 (The Royal Society of Chemistry, 2009).
Serp, P. & Kalck, P. Chemical vapor deposition methods for the controlled preparation of supported catalytic materials. Chem. Rev. 102, 3085–3128 (2002).
George, S. M. Atomic layer deposition: an overview. Chem. Rev. 110, 111–131 (2010).
Lefévre, M., Proietti, E., Jaouen, F. & Dodelet, J-P. Iron-based catalysts with improved oxygen reduction activity in polymer electrolyte fuel cells. Science 324, 71–74 (2009).
Lara, P. et al. Ruthenium nanoparticles stabilized by N-heterocyclic carbenes: ligand location and influence on reactivity. Angew. Chem. Int. Ed. 50, 12080–12084 (2011).
Bronger, R. et al. Multi-site coordination N-phosphanylamidine ligands as stabilizers for the synthesis of ruthenium nanoparticles. New J. Chem. 35, 2653–2660 (2011).
Guerrero, M. et al. Design of new N,O hybrid pyrazole derived ligands and their use as stabilizers for the synthesis of Pd-nanoparticles. Langmiur 26, 15532–15540 (2010).
Downing, R. S., Kunkeler, P. J. & van Bekkum, H. Catalytic syntheses of aromatic amines. Catal. Today 37, 121–136 (1997).
Ono, N. The Nitro Group in Organic Synthesis (Wiley-VCH, 2001).
Suchy, M., Winternitz, P. & Zeller, M. Heterocyclic compounds. WO patent 91/00278 (1991).
Butera, J. & Bagli, J. n-Heteroaralkyl-substituted 1-aryloxy-2-propanolamine and propylamine derivatives processing class III antiarrythmic activity. WO patent 91/09023 (1991).
Kolvar, R. F. & Armond, E. F. Ethynyl-substituted aromatic ortho diamines and method of synthesis. US patent 3,975,444 (1976).
Reis, P. M. & Royo, B. Chemoselective hydrogenation of nitroarenes and deoxygenation of pyridine N-oxides with H2 catalyzed by MoO2Cl2 . Tetrahedron Lett. 50, 949–952 (2009).
Liu, L., Qiao, B., Chen, Z., Zhang, J. & Deng, Y. Novel chemoselective hydrogenation of aromatic nitro compounds over ferric hydroxide supported nanocluster gold in the presence of CO and H2O. Chem. Commun. 653–655 (2009).
Cao, S., Xu, S. & Xu, S. Hydrogenation of nitroaromatics containing a carbonyl group catalyzed by the palladium complex of MgO-supported melamino-formaldehyde polymer. Polym. Adv. Technol. 10, 43–47 (1999).
Boix, C. & Poliakoff, M. Selective reductions of nitroarenes to anilines using metallic zinc in near-critical water. J. Chem. Soc. Perkin Trans. 1, 1487–1490 (1999).
Cardenas-Lizana, F. et al. Pd-promoted selective gas phase hydrogenation of p-chloronitrobenzene over alumina supported Au. J. Catal. 262, 235–243 (2009).
Cardenas-Lizana, F., Gomez-Quero S. & Keane, M. A. Exclusive production of chloroaniline from chloronitrobenzene over Au/TiO2 and Au/Al2O3 . ChemSusChem 1, 215–221 (2008).
Ichikawa, S., Tada, M., Iwasawab, Y. & Ikariya, T. The role of carbon dioxide in chemoselective hydrogenation of halonitroaromatics over supported noble metal catalysts in supercritical carbon dioxide. Chem. Commun. 924–926 (2005).
Knifton, J. F. Homogenous catalyzed reduction of nitro-compounds for selective and sequential hydrogenation of nitroaromatics. J. Org. Chem. 41, 1200–1206 (1976).
Joshi, R. & Chudasama, U. Hydrogenation and oxidation reactions involving ruthenium supported catalysts. Ind. Eng. Chem. Res. 49, 2543–2547 (2010).
Deshmukh, A. A., Prashar, A. K., Kinage, A. K., Kumar, R. & Meijboom, R. Ru(II)– phenanthroline complex as catalyst for chemoselective hydrogenation of nitro-aryls in a green process. Ind. Eng. Chem. Res. 49, 12180–12184 (2010).
Savoia, D., Trombini, C., Umani-Ronchi, A. & Verardo, G. Active metals from potassium–graphite. Palladium–graphite as catalyst in the hydrogenation of nitro-compounds, alkenes, and alkynes. J. Chem. Soc. Chem. Comm. 540–541 (1981).
Tafesh, A. M. & Beller, M. First selective reduction of aromatic nitro compounds using water soluble catalysts. Tetrahedron Lett. 36, 9305–9308 (1995).
Onopchenko, A., Sabourin, E. T. & Selwitz, C. M. Selective catalytic-hydrogenation of aromatic nitro-groups in the presence of acetylenes – synthesis of (3-aminophenyl)acetylene via hydrogenation of dimethycarbinol substituted (3-nitrophenyl)acetylene over heterogeneous metallic ruthenium catalysts. J. Org. Chem. 44, 1233–1236 (1979).
Siegrist, U., Baumeister, P. & Blaser, H-U. in Catalysis of Organic Reactions (ed. Herkes, F.) 207–219 (Chemical Industries Series 75, Marcel Dekker, 1998).
Raja, R. et al. Highly efficient catalysts for the hydrogenation of nitro-substituted aromatics. Chem. Commun. 2026–2028 (2005).
Braden, R., Knupfer H. & Hartung, S. Process for the preparation of unsaturated amino compounds. US patent 4,002,673 (1977).
Blaser, H-U., Siegrist, U., Steiner, H. & Studer, M. in Aromatic Nitro Compounds: Fine Chemicals through Heterogeneous Catalysis (eds Sheldon, R. A. & van Bekkum, H.) 389–406 (Wiley-VCH, 2001).
Blaser, H-U., Steiner, H. & Studer, M. Selective catalytic hydrogenation of functionalized nitroarenes: an update. ChemCatChem 1, 210–221 (2009).
Corma, A. & Serna, P. Chemoselective hydrogenation of nitro compounds with supported gold catalysts. Science 313, 332–334 (2006).
Corma, A., Gonález-Arellano, C., Iglesias, M. & Sánchez, F. Gold complexes as catalysts: chemoselective hydrogenation of nitroarenes. Appl. Catal. A 356, 99–102 (2009).
Corma, A., Serna, P., Concepción, P. & Calvino, J. Transforming nonselective into chemoselective metal catalysts for the hydrogenation of substituted nitroaromatics. J. Am. Chem. Soc. 130, 8748–8753 (2008).
Wienhöfer, G. et al. General and selective iron-catalyzed transfer hydrogenation of nitroarenes without base. J. Am. Chem. Soc. 133, 12875–12879 (2011).
Angelov, S., Zhecheva, E., Stoyanova, R. & Atanasov, M. Bulk defects in Co3O4, pure and slightly doped with lithium, revealed by EPR of the tetrahedral CO2+ ions. J. Phys. Chem. Solids 51, 1157–1161 (1990).
Dutta, P., Seehra, M. S. Thota, S. & Kumar, J. A comparative study of the magnetic properties of bulk and nanocrystalline Co3O4 . J. Phys. Condens. Matter 20, 015218 (2008).
Brückner, A., Martin, A., Steinfeldt, N., Wolf, G-U. & Lücke, B. Investigation of vanadium phosphorus oxide catalysts (VPO) during toluene ammoxidation: new mechanistic insights by in situ EPR. J. Chem. Soc. Faraday Trans. 92, 4257–4263 (1996).
Wdowik, U. D. & Legut, D. CoO under pressure from first principles. J. Phys. Chem. Solids 69, 1698–1703 (2008).
Papaefthimiou, V. et al. Nontrivial redox behavior of nanosized cobalt: new insights from ambient pressure X-ray photoelectron and absorption spectroscopies. ACS Nano 5, 2182–2190 (2011).
Herranz, T., Deng, X. Y. Cabot, A., Guo, J. G. & Salmeron, M. Influence of the cobalt particle size in the Co hydrogenation reaction studied by in situ X-ray absorption spectroscopy. J. Phys. Chem. B 113, 10721–10727 (2009).
Bezemer, G. L. et al. Cobalt particle size effects in the Fischer–Tropsch reaction studied with carbon nanofiber supported catalysts. J. Am. Chem. Soc. 128, 3956–3964 (2006).
Casanovas, J., Ricart, J. M., Rubio, J., Illas, F. & Jiménez-Mateos, J. M. Origin of the large N 1s binding energy in X-ray photoelectron spectra of calcined carbonaceous materials. J. Am. Chem. Soc. 118, 8071–8076 (1996).
Pels, J. R., Kapteijn, F., Moulijn, J. A., Zhu, Q. & Thomas, K. M. Evolution of nitrogen functionalities in carbonaceous material during pyrolysis. Carbon 33, 1641–1653 (1995).
Grünert, W. et al. A new facility for inert transfer of reactive samples to XPS equipment. J. Electron. Spectrosc. Relat. Phenom. 40, 187–192 (1986).
Simon, M. O. & Li, C. J. Green chemistry oriented organic synthesis in water. Chem. Soc. Rev. 4, 1415–1427 (2012).
de Cienfuegos, L. A., Robles, R., Miguel, D., Justicia, J. & Cuerva, J. M. Reduction reactions in green solvents: water, supercritical carbon dioxide, and ionic liquids. ChemSusChem 8, 1035–1048 (2011).
Marson, C. M. New and unusual scaffolds in medicinal chemistry. Chem. Soc. Rev. 40, 5514–5533 (2011).
Cornils, B. & Herrmann, W. A. Applied Homogeneous Catalysis with Organometallic Compounds (Wiley-VCH, 2002).
Colman, J. P., Hegedus, L. S., Norton, J. R. & Finke, R. G. Principles and Applications of Organotransition Metal Chemistry (University Science Books, 1987).
Corma, A. & Garcia, H. Engineering metal organic frameworks for heterogeneous catalysis. Chem. Rev. 8, 4606–4655 (2010).
Dioos, B., Vankelecom, I. & Jacobs, P. Aspects of immobilisation of catalysts on polymeric supports. Adv. Synth. Catal. 348, 1413–1446 (2006).
Collins, A. & Horvath, I. Heterogenization of homogeneous catalytic systems. Catal. Sci. Tech. 1, 912–919 (2011).
Acknowledgements
This research was supported by Federal Ministry of Education and Research (BMBF) and the state of Mecklenburg-Vorpommern.
Author information
Authors and Affiliations
Contributions
M.B., F.A.W. and R.V.J. planned and developed the project, F.A.W. performed and designed the experiments and co-wrote the paper, R.V.J. developed, designed and prepared the catalysts and was involved in writing the paper, G.W. conducted catalytic experiments and performed the analytics, M.N. was involved in discussions and contributed to the writing of the paper, K.J. participated in discussions and contributed to the writing of the paper, H.J. was involved in the development of the catalyst, A.E.S. performed electrochemical studies of the catalyst and was involved in the catalyst development, J. Radnik did the XPS experiments, M.M.P. performed the TEM experiments, J. Rabeah and A.B. were responsible for EPR experiments, M.B. directed the project, participated in the design of catalytic experiments and in the design of the catalyst, and co-wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 1050 kb)
Rights and permissions
About this article
Cite this article
Westerhaus, F., Jagadeesh, R., Wienhöfer, G. et al. Heterogenized cobalt oxide catalysts for nitroarene reduction by pyrolysis of molecularly defined complexes. Nature Chem 5, 537–543 (2013). https://doi.org/10.1038/nchem.1645
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.1645
This article is cited by
-
Enhancing graphitization and mesoporosity by cobalt in activated carbons obtained from peach stone
Journal of Nanoparticle Research (2024)
-
Oxidative cleavage and ammoxidation of organosulfur compounds via synergistic Co-Nx sites and Co nanoparticles catalysis
Nature Communications (2023)
-
Efficient reduction of quinoxaline compounds using zinc chloride—sodium borohydride system
Russian Chemical Bulletin (2023)
-
Solvent-free synthesis of Co@NC catalyst with Co—N species as active sites for chemoselective hydrogenation of nitro compounds
Science China Materials (2023)
-
ZIF-8@ZIF-67 Derived Co/NPHC Catalysts for Efficient and Selective Hydrogenation of Nitroarenes
Catalysis Letters (2023)