Abstract
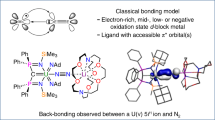
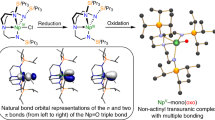
The nature and extent of covalency in uranium bonding is still unclear compared with that of transition metals, and there is great interest in studying uranium–ligand multiple bonds. Although U=O and U=NR double bonds (where R is an alkyl group) are well-known analogues to transition-metal oxo and imido complexes, the uranium(VI)–nitride triple bond has long remained a synthetic target in actinide chemistry. Here, we report the preparation of a uranium(VI)–nitride triple bond. We highlight the importance of (1) ancillary ligand design, (2) employing mild redox reactions instead of harsh photochemical methods that decompose transiently formed uranium(VI) nitrides, (3) an electrostatically stabilizing sodium ion during nitride installation, (4) selecting the right sodium sequestering reagent, (5) inner versus outer sphere oxidation and (6) stability with respect to the uranium oxidation state. Computational analyses suggest covalent contributions to U≡N triple bonds that are surprisingly comparable to those of their group 6 transition-metal nitride counterparts.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Change history
10 May 2013
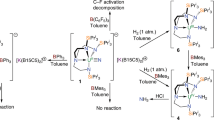
In the version of this Article originally published online, in the second paragraph of the section 'Synthetic considerations for preparing U≡N triple bonds', compound 7 was referred to instead of compound 12. The relevant text should have read 'The characterization data for 12 fully support its formulation (Supplementary Information). The crystal structure of 12 (Fig. 2c) reveals...' This has been corrected in the HTML and PDF versions of the Article.
References
Nugent, W. A. & Mayer, J. M. Metal–Ligand Multiple Bonds (Wiley, 1988).
Giesbrecht, G. R. & Gordon, J. C. Lanthanide alkylidene and imido complexes. Dalton Trans. 2387–2393 (2004).
Summerscales, O. T. & Gordon, J. C. Complexes containing multiple bonding interactions between lanthanoid elements and main-group fragments. RSC Adv. http://dx.doi.org/10.1039/C3RA23151H (2013).
Ephritikhine, M. The vitality of uranium molecular chemistry at the dawn of the XXIst century. Dalton Trans. 2501–2516 (2006).
Hayton, T. W. Metal–ligand multiple bonding in uranium: structure and reactivity. Dalton Trans. 39, 1145–1158 (2010).
Hayton, T. W. Recent developments in actinide–ligand multiple bonding. Chem. Commun. 49, 2956–2973 (2013).
Castro-Rodríguez, I. & Meyer, K. Small molecule activation at uranium coordination complexes: control of reactivity via molecular architecture. Chem. Commun. 1353–1368 (2006).
Lam, O. P., Anthon, C. & Meyer, K. Influence of steric pressure on the activation of carbon dioxide and related small molecules by uranium coordination complexes. Dalton Trans. 9677–9691 (2009).
Fox, A. R., Bart, S. C., Meyer, K. & Cummins, C. C. Towards uranium catalysts. Nature 455, 341–349 (2008).
Cooper, O. J. et al. Uranium–carbon multiple bonding: facile access to the pentavalent uranium carbene [U{C(PPh2NSiMe3)2}(Cl)2(I)] and comparison of UV=C and UIV=C double bonds. Angew. Chem. Int. Ed. 50, 2383–2386 (2011).
Mills, D. P. et al. Synthesis of a uranium(VI) carbene: reductive formation of uranyl(V) methanides, oxidative preparation of a [R2C=U=O]2+ analogue of the [O=U=O]2+ uranyl ion (R = Ph2PNSiMe3), and comparison of the nature of UIV=C, UV=C and UVI=C double bonds. J. Am. Chem. Soc. 134, 10047–10054 (2012).
Cantat, T. et al. The U=C double bond: synthesis and study of uranium nucleophilic carbene complexes. J. Am. Chem. Soc. 131, 963–972 (2009).
Tourneux, J-C. et al. Exploring the uranyl organometallic chemistry: from single to double uranium–carbon bonds. J. Am. Chem. Soc. 133, 6162–6165 (2011).
Fortier, S., Walensky, J. R., Wu, G. & Hayton, T. W. Synthesis of a phosphorano-stabilized U(IV) carbene via one-electron oxidation of a U(III)–ylide adduct. J. Am. Chem. Soc. 133, 6894–6897 (2011).
Brown, J. L., Fortier, S., Lewis, R. A., Wu, G. & Hayton, T. W. A complete family of terminal uranium chalcogenides, [U(E)(N{SiMe3}2)3]− (E = O, S, Se, Te). J. Am. Chem. Soc. 134, 15468–15475 (2012).
Streit, M. & Ingold, F. Nitrides as a nuclear fuel option. J. Eur. Ceram. Soc. 25, 2687–2692 (2005).
Chinthaka Silva, G. W. et al. Reaction sequence and kinetics of uranium nitride decomposition. Inorg. Chem. 48, 10635–10642 (2009).
Kempter, C. P., McGuire, J. C. & Nadler, M. R. Uranium mononitride. Anal. Chem. 31, 156–157 (1959).
Green, D. W. & Reedy, G. T. The identification of UN in Ar matrices. J. Chem. Phys. 65, 2921–2922 (1976).
Hunt, R. D., Yustein, J. T. & Andrews, L. Matrix infrared spectra of NUN formed by the insertion of uranium atoms into molecular nitrogen. J. Chem. Phys. 98, 6070–6074 (1993).
Andrews, L., Wang, X., Lindh, R., Roos, B. O. & Marsden, C. J. Simple N≡UF3 and P≡UF3 molecules with triple bonds to uranium. Angew. Chem. Int. Ed. 47, 5366–5370 (2008).
Wang, X., Andrews, L., Vlaisavljevich, B. & Gagliardi, L. Combined triple and double bonds to uranium: the N≡U=N–H uranimine nitride molecule prepared in solid argon. Inorg. Chem. 50, 3826–3831 (2011).
Zhou, M. & Andrews, L. Infrared spectra and pseudopotential calculations for NUO+, NUO, and NThO in solid neon. J. Chem. Phys. 111, 11044–11049 (1999).
Heinemann, C. & Schwarz, H. NUO+, a new species isoelectronic to the uranyl dication UO22+. Chem. Eur. J. 1, 7–11 (1995).
Pyykkö, P., Li, J. & Runeberg, N. Quasirelativistic pseudopotential study of species isoelectronic to uranyl and the equatorial coordination of uranyl. J. Phys. Chem. 98, 4809–4813 (1994).
Korobkov, I., Gambarotta, S. & Yap, G. P. A. A highly reactive uranium complex supported by the calix[4]tetrapyrrole tetraanion affording dinitrogen cleavage, solvent deoxygenation, and polysilanol depolymerization. Angew. Chem. Int. Ed. 41, 3433–3436 (2002).
Evans, W. J., Kozimor, S. A. & Ziller, J. W. Molecular octa-uranium rings with alternating nitride and azide bridges. Science 309, 1835–1838 (2005).
Evans, W. J., Miller, K. A., Ziller, J. W. & Greaves, J. Analysis of uranium azide and nitride complexes by atmospheric pressure chemical ionization mass spectrometry. Inorg. Chem. 46, 8008–8018 (2007).
Nocton, G., Pécaut, J. & Mazzanti, M. A nitrido-centered uranium azido cluster obtained from a uranium azide. Angew. Chem. Int. Ed. 47, 3040–3042 (2008).
Fox, A. R., Arnold, P. L. & Cummins, C. C. Uranium–nitrogen multiple bonding: isostructural anionic, neutral, and cationic uranium nitride complexes featuring a linear U=N=U core. J. Am. Chem. Soc. 132, 3250–3251 (2010).
Fortier, S., Wu, G. & Hayton, T. W. Synthesis of a nitrido-substituted analogue of the uranyl ion, [N=U=O]+. J. Am. Chem. Soc. 132, 6888–6889 (2010).
Fox, A. R. & Cummins, C. C. Uranium–nitrogen multiple bonding: the case of a four-coordinate uranium(VI) nitridoborate complex. J. Am. Chem. Soc. 131, 5716–5717 (2009).
Thomson, R. K. et al. Uranium azide photolysis results in C–H bond activation and provides evidence for a terminal uranium nitride. Nature Chem. 2, 723–729 (2010).
King, D. M. et al. Synthesis and structure of a terminal uranium nitride complex. Science 337, 717–720 (2012).
Denning, R. G. Electronic structure and bonding in actinyl ions. Struct. Bonding (Berl.) 79, 215–276 (1992).
Kaltsoyannis, N. Computational study of analogues of the uranyl ion containing the –N=U=N– unit: density functional theory calculations on UO22+, UON+, UN2, UO(NPH3)3+, U(NPH3)24+, [UCl4{NPR3}2] (R = H, Me), and [UOCl4{NP(C6H5)3}]−. Inorg. Chem. 39, 6009–6017 (2000).
Kosog, B., La Pierre, H. S., Heinemann, F. W., Liddle, S. T. & Meyer, K. Synthesis of uranium(VI) terminal oxo complexes: molecular geometry driven by the inverse trans-influence. J. Am. Chem. Soc. 134, 5284–5289 (2012).
Mills, D. P. et al. A delocalized arene-bridged diuranium single-molecule magnet. Nature Chem. 3, 454–460 (2011).
Lam, O. P., Heinemann, F. W. & Meyer, K. Activation of elemental S, Se and Te with uranium(III): bridging U–E–U (E = S, Se) and diamond-core complexes U–(E)2–U (E = O, S, Se, Te). Chem. Sci. 2, 1538–1547 (2011).
Kramer, G. M., Dines, M. B., Kaldor, A., Hall, R. & McClure, D. Photochemical behavior of a uranyl bis(hexafluoroacetylacetonate)–tetrahydrofuran complex. 1. Inorg. Chem. 20, 1421–1426 (1981).
Natrajan, L. S. Developments in the photophysics and photochemistry of actinide ions and their coordination compounds. Coord. Chem. Rev. 256, 1583–1603 (2012).
Zi, G. et al. Preparation and reactions of base-free bis(1,2,4-tri-tert-butylcyclopentadienyl)uranium oxide, Cp′2UO. Organometallics 24, 4251–4264 (2005).
Bailey, P. J. et al. The first structural characterisation of a group 2 metal alkylperoxide complex: comments on the cleavage of dioxygen by magnesium alkyl complexes. Chem. Eur. J. 9, 4820–4828 (2003).
Graves, C. R. & Kiplinger, J. L. Pentavalent uranium chemistry – synthetic pursuit of a rare oxidation state. Chem. Commun. 3831–3853 (2009).
Hayton, T. W. et al. Synthesis of imido analogs of the uranyl ion. Science 310, 1941–1943 (2005).
Zalkin, A., Brennan, J. G. & Andersen, R. A. Tris[bis(trimethylsilyl)amido](trimethylsilylimido)uranium(V). Acta Cryst. C 44, 1553–1554 (1988).
Burns, C. J., Smith, W. H., Huffman, J. C. & Sattelberger, A. P. Uranium(VI) organoimido complexes. J. Am. Chem. Soc. 112, 3237–3239 (1990).
Arney, D. S., Burns, C. J. & Smith, D. C. Synthesis and structure of the first uranium(VI) organometallic complex. J. Am. Chem. Soc. 114, 10068–10069 (1992).
Odom, A. L. & Cummins, C. C. Nitric oxide cleavage: synthesis of terminal chromium(VI) nitride complexes via nitrosyl deoxygenation. J. Am. Chem. Soc. 117, 6613–6614 (1995).
Curley, J. J., Cook, T. R., Reece, S. Y., Müller, P. & Cummins, C. C. Shining light on dinitrogen cleavage: structural features, redox chemistry, and photochemistry of the key intermediate bridging dinitrogen complex. J. Am. Chem. Soc. 130, 9394–9405 (2008).
Clough, C. R. et al. Organic nitriles from acid chlorides: an isovalent N for (O)Cl exchange reaction mediated by a tungsten nitride complex. J. Am. Chem. Soc. 126, 7742–7743 (2004).
Pepper, M. & Bursten, B. E. The electronic structure of actinide-containing molecules: a challenge to applied quantum chemistry. Chem. Rev. 91, 719–741 (1991).
Acknowledgements
We are grateful to the European Research Council, the UK Engineering and Physical Sciences Research Council, including the National UK Electron Paramagnetic Resonance Facility at Manchester, the University of Nottingham and the UK National Nuclear Laboratory for generous funding and support, the Royal Society for the award of a University Research Fellowship (S.T.L.) and European Cooperation in Science and Technology (COST) Action CM1006 for support.
Author information
Authors and Affiliations
Contributions
D.M.K. synthesized and characterized the compounds. F.T. and E.J.L.M. recorded and analysed the electron paramagnetic resonance data. J.M. carried out and analysed the DFT calculations. W.L. and A.J.B. carried out the X-ray single-crystal structure analyses. S.T.L. originated the central idea, supervised the work, analysed the data and wrote the manuscript with contributions from all the co-authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 2727 kb)
Crystallographic data for compound 6
(CIF 53 kb)
Crystallographic data for compound 7
(CIF 61 kb)
Crystallographic data for compound 8
(CIF 33 kb)
Crystallographic data for compound 9
(CIF 31 kb)
Crystallographic data for compound 10
(CIF 33 kb)
Crystallographic data for compound 11
(CIF 49 kb)
Crystallographic data for compound 12
(CIF 30 kb)
Crystallographic data for compound 13
(CIF 35 kb)
Rights and permissions
About this article
Cite this article
King, D., Tuna, F., McInnes, E. et al. Isolation and characterization of a uranium(VI)–nitride triple bond. Nature Chem 5, 482–488 (2013). https://doi.org/10.1038/nchem.1642
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.1642
This article is cited by
-
Actinide inverse trans influence versus cooperative pushing from below and multi-center bonding
Nature Communications (2023)
-
Prospects and challenges for nitrogen-atom transfer catalysis
Nature Reviews Chemistry (2023)
-
Accessing five oxidation states of uranium in a retained ligand framework
Nature Communications (2023)
-
A charged diatomic triple-bonded U≡N species trapped in C82 fullerene cages
Nature Communications (2022)
-
Photochemical Synthesis of Transition Metal-Stabilized Uranium(VI) Nitride Complexes
Nature Communications (2022)