Abstract
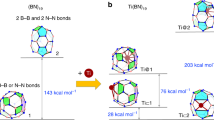
Only a handful of elements are able to be controllably homocatenated (that is, to be formed into one- or two-dimensional chains or rings of the element), because most have weak element–element bonds. Boron forms strong B–B bonds, but its favourable cluster formation makes homocatenation very difficult. Recently, the coupling of borylene (:BR) ligands on a metal was predicted computationally. We have brought this prediction to fruition experimentally, and extended it by adding two further borylene units, stepwise forming a B4 chain bound to a metal under mild conditions. This complex is a useful model for understanding the metal–boron interactions required to promote transition of the boron atoms from borylene ligands to oligoborane networks bound side-on. The concept shows great promise for the controlled construction of one-dimensional boron chains.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Pace, N. R. The universal nature of biochemistry. Proc. Natl Acad. Sci. USA 98, 805–808 (2001).
Huheey, J. E., Keiter, E. A. & Keiter, R. L. Inorganic Chemistry 4th edn (Harper, 1995).
Miller, R. D. & Michl, J. Polysilane high polymers. Chem. Rev. 107, 1359–1410 (1989).
Urry, G., Garrett, A. G. & Schlesinger, H. I. The chemistry of the boron subhalides. I. Some properties of tetraboron tetrachloride, B4Cl4 . Inorg. Chem. 2, 396–400 (1963).
Hermannsdörfer, K. H., Matejčikova, E. & Nöth, H. Dimethylamino-polyborane. Chem. Ber. 103, 516–527 (1970).
Nöth, H. & Pommerening, H. Hexakis(dimethylamino)cyclohexaborane, a boron(I) compound without electron deficiency. Angew. Chem. Int. Ed. 19, 482–483 (1980).
Klusik, H. & Berndt, A. The radical anion from tetra-t-butyltetraborane(4), a new route to t-Bu4B4 . J. Organomet. Chem. 234, C17–C19 (1982).
Baudler, M., Rockstein, K. & Oehlert, W. Tris(diethylamino)cyclotriborane and constitutional isomerism between cyclo- and closo-hexakis(diethylamino)hexaborane(6). Chem. Ber. 124, 1149–1152 (1991).
Linti, G., Loderer, D., Nöth, H., Polborn, K. & Rattay, W. Reactions and structure of electron-precise triborane(5) and tetraborane(6) derivatives. Chem. Ber. 127, 1909–1922 (1994).
Maier, C-J., Pritzkow, H. & Siebert, W. Blue tetrakis(diisopropylamino)-cyclo-tetraborane and yellow tetrakis(tetramethylpiperidino)tetrabora-tetrahedrane. Angew. Chem. Int. Ed. 38, 1666–1668 (1999).
Kleier, D. A., Bicerano, J. & Lipscomb, W. N. Stereochemical rigidity and isomerization in B4H4 and B4F4. A theoretical study. Inorg. Chem. 19, 216–218 (1980).
Davan, T. & Morrison, J. A. Tetrakis(t-butyl)tetraborane(4), Bu4tB4; synthesis of the first peralkyl derivative of a 2N framework electron count deltahedral borane. J. Chem. Soc. Chem. Commun. 250–251 (1981).
Morrison, J. A. Chemistry of the polyhedral boron halides and the diboron tetrahalides. Chem. Rev. 91, 35–48 (1991).
Menneckes, T., Paetzold, P., Boese, R. & Bläser, D. Tetra-tert-butyltetraboratetrahedrane. Angew. Chem. Int. Ed. 30, 173–175 (1991).
Neu, A. et al. Tetra-tert-butyltetraborane(6) B4H2tBu4: a derivative in the series BnHn +2 . Angew. Chem. Int. Ed. 36, 2117–2119 (1997).
Schnepf, A., Doriat, C., Möllhausen, E. & Schnöckel, H. A simple synthesis for donor-stabilized Ga2I4 and Ga3I5 species and the X-ray crystal structure of Ga3I5·3PEt3 . Chem. Commun. 2111–2112 (1997).
Brothers, P. J. et al. A new In4 cluster with short In–In bonds in trigonal-planar In(InTrip2)3 . Angew. Chem. Int. Ed. 35, 2355–2357 (1996).
Hill, M. S., Hitchcock, P. B. & Pongtavornpinyo, R. A linear homocatenated compound containing six indium centers. Science 311, 1904–1907 (2006).
Green, S. P., Jones, C. & Stasch, A. ‘Dissolution’ of indium(I) iodide: synthesis and structural characterization of the neutral indium sub-halide cluster complex [In6I8(tmeda)4]. Angew. Chem. Int. Ed. 46, 8618–8621 (2007).
Braunschweig, H., Kollann, C. & Rais, D. Transition-metal complexes of boron – new insights and novel coordination modes. Angew. Chem. Int. Ed. 45, 5254–5274 (2006).
Braunschweig, H., Kollann, C. & Seeler, F. Transition metal borylene complexes. Struct. Bond. 130, 1–27 (2008).
Braunschweig, H., Dewhurst, R. D. & Schneider, A. Electron-precise coordination modes of boron-centered ligands. Chem. Rev. 110, 3924–3957 (2010).
Bissinger, P., Braunschweig, H., Kraft, K. & Kupfer, T. Trapping the elusive parent borylene. Angew. Chem. Int. Ed. 50, 4704–4707 (2011).
Kinjo, R., Donnadieu, B., Celik, M. A., Frenking, G. & Bertrand, G. Synthesis and characterization of a neutral tricoordinate organoboron isoelectronic with amines. Science 333, 610–613 (2011).
Bissinger, P. et al. Generation of a carbene-stabilized bora-borylene and its insertion into a C–H bond. J. Am. Chem. Soc. 133, 19044–19047 (2011).
Braunschweig, H., Colling, M., Hu, C. & Radacki, K. From classical to nonclassical metal–boron bonds: synthesis of a novel metallaborane. Angew. Chem. Int. Ed. 41, 1359–1361 (2002).
Xu, L., Li, Q., King, R. B. & Schaefer, H. F. III. Coupling of fluoroborylene ligands to give a viable cyclopentadienyliron carbonyl complex of difluorodiborene (FB=BF). Organometallics 30, 5084–5087 (2011).
Bertsch, S. et al. Towards homoleptic borylene complexes: incorporation of two borylene ligands into a mononuclear iridium species. Angew. Chem. Int. Ed. 49, 9517–9520 (2010).
Braunschweig, H., Ye, Q. & Radacki, K. High yield synthesis of a neutral and carbonyl-rich terminal arylborylene complex. Chem. Commun. 48, 2701–2703 (2012).
Braunschweig, H. et al. Borylene-based functionalization of iron-alkynyl-σ-complexes and stepwise reversible metal-boryl-to-borirene transformation: synthesis, characterization, and density functional theory studies. Inorg. Chem. 50, 62–71 (2011).
Braunschweig, H. et al. Synthesis of 1-aza-2-borabutatriene rhodium complexes by thermal borylene transfer from [(OC)5Mo=BN(SiMe3)2]. Angew. Chem. Int. Ed. 50, 9462–9466 (2011).
Braunschweig, H. et al. Borylene-based direct functionalization of organic substrates: synthesis, characterization, and photophysical properties of novel π-conjugated borirenes. J. Am. Chem. Soc. 131, 8989–8999 (2009).
Braunschweig, H., Herbst, T., Rais, D. & Seeler, F. Synthesis of borirenes by photochemical borylene transfer from [(OC)5M=BN(SiMe3)2] (M = Cr, Mo) to alkynes. Angew. Chem. Int. Ed. 44, 7461–7463 (2005).
Braunschweig, H., Forster, M., Kupfer, T. & Seeler, F. Borylene transfer under thermal conditions for the synthesis of rhodium and iridium borylene complexes. Angew. Chem. Int. Ed. 47, 5981–5983 (2008).
Bellachioma, G., Cardaci, G., Macchioni, A. & Reichenbach, G. Preparation and characterization by 31P-NMR spectroscopy of mixed disubstituted [Fe(CO)3LL′] complexes. J. Organomet. Chem. 391, 367–376 (1990).
Poliakoff, M. & Turner, J. J. Infrared spectrum and photochemistry of di-iron enneacarbonyl in matrices at 20 K: evidence for the formation of Fe2(CO)8 . J. Chem. Soc. A 2403–2410 (1971).
Fletcher, S. C., Poliakoff, M. & Turner, J. J. Structure and reactions of octacarbonyldiiron: an IR spectroscopic study using carbon-13 monoxide, photolysis with plane-polarized light, and matrix isolation. Inorg. Chem. 25, 3597–3604 (1986).
Fedrigo, S., Haslett, T. L. & Moskovits, M. Direct synthesis of metal cluster complexes by deposition of mass-selected clusters with ligand: iron with CO. J. Am. Chem. Soc. 118, 5083–5085 (1996).
Cotton, F. A. & Troup, M. J. Accurate determination of a classic structure in the metal carbonyl field: nonacarbonyldi-iron. J. Chem. Soc. Dalton Trans. 800–802 (1974).
Perrin, D. D. & Armarego, W. L. F. Purification of Laboratory Chemicals 3rd edn (Pergamon, 1988).
Blank, B. et al. Aminoborylene complexes of group 6 elements and iron: a synthetic, structural, and quantum chemical study. Chem. Eur. J. 13, 4770–4781 (2007).
Acknowledgements
Financial support from the European Research Council (Advanced Investigator Grant to H.B.) is acknowledged.
Author information
Authors and Affiliations
Contributions
H.B. conceived and supervised the study, Q.Y. performed the syntheses, A.V. performed the computational experiments and K.R. and A.D. performed the X-ray crystallographic measurements. R.D.D., Q.Y. and A.V. analysed the data and co-wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 658 kb)
Supplementary information
Crystallographic data for compound 2. (CIF 19 kb)
Supplementary information
Crystallographic data for compound 3. (CIF 20 kb)
Supplementary information
Crystallographic data for compound 4. (CIF 31 kb)
Rights and permissions
About this article
Cite this article
Braunschweig, H., Ye, Q., Vargas, A. et al. Controlled homocatenation of boron on a transition metal. Nature Chem 4, 563–567 (2012). https://doi.org/10.1038/nchem.1379
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.1379
This article is cited by
-
Catalysis in service of main group chemistry offers a versatile approach to p-block molecules and materials
Nature Chemistry (2013)
-
A boron–boron coupling reaction between two ethyl cation analogues
Nature Chemistry (2013)
-
Bond-strengthening π backdonation in a transition-metal π-diborene complex
Nature Chemistry (2013)
-
Metal-reinforced bonding
Nature Chemistry (2013)