Abstract
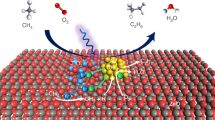
Catalytic hydrogen production from renewables is a promising method for providing energy carriers in the near future. Photocatalysts capable of promoting this reaction are often composed of noble metal nanoparticles deposited on a semiconductor. The most promising semiconductor at present is TiO2. The successful design of these catalysts relies on a thorough understanding of the role of the noble metal particle size and the TiO2 polymorph. Here we demonstrate that Au particles in the size range 3–30 nm on TiO2 are very active in hydrogen production from ethanol. It was found that Au particles of similar size on anatase nanoparticles delivered a rate two orders of magnitude higher than that recorded for Au on rutile nanoparticles. Surprisingly, it was also found that Au particle size does not affect the photoreaction rate over the 3–12 nm range. The high hydrogen yield observed makes these catalysts promising materials for solar conversion.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Xiaofeng, L. & Goodman, D. W. Structure–reactivity correlations for oxide-supported metal catalysts: new perspectives from STM. J. Mol. Catal. A 162, 33–50 (2000).
Chen, M. & Goodman, D. W. Catalytically active gold on ordered titania supports. Chem. Soc. Rev. 37, 1860–1870 (2008).
Bamwenda, G. R., Tsubota, S., Nakamura, T. & Haruta, M. Photoassisted hydrogen production from a water-ethanol solution: a comparison of activities of Au-TiO2 and Pt-TiO2 . J. Photochem. Photobiol. A 89, 177–189 (1995).
Li, X. Z. & Li, F. B. Study of Au/Au3+-TiO2 photo-catalysts toward visible photo-oxidation for water and wastewater treatment. Environ. Sci. Technol. 35, 2381–2387 (2001).
Al-Mazroai, L. S. et al. The photocatalytic reforming of methanol. Catal. Today 122, 46–50 (2007).
Yang, Z., Chang, C-H. & Idriss, H. Photo-catalytic production of hydrogen form ethanol over M/TiO2 catalysts (M=Pd, Pt or Rh). Appl. Catal. B 67, 217–222 (2006).
Fujishima, A., Zhang, X. & Tryk, D. A. TiO2 photocatalysis and related surface phenomena. Surf. Sci. Rep., 63, 515–582 (2008).
Cárdenas-Lizana, F., Gómez-Quero, S., Idriss, H. & Keane, M. A. Gold particle size effects in the gas-phase hydrogenation of m-dinitrobenzene over Au/TiO2 . J. Catal. 268, 223–234 (2009).
Kamat, P. V. Quantum dot solar cells. Semiconductor nanocrystals as light harvesters. J. Phys. Chem. C, 112, 18737–18753 (2008).
Valden, M., Lai, X. & Goodman, D. W. Onset of catalytic activity of gold clusters on titania with the appearance of nonmetallic properties. Science 281, 1647–1650 (1998).
Moulder, J. F., Stickle, W. F., Sobol, P. E. & Bomben, K. D. in Handbook of X-ray photoelectron spectroscopy (Eden Prairie, 1992).
Hashmi, A. S. K. & Hutchings, G. J. Gold catalysis. Angew. Chem. Int. Ed. 45, 7896–7936 (2006).
Nadeem, M. A. et al. Photoreaction of ethanol on Au/TiO2 anatase: Comparing the micro to nanoparticle size activities of the support for hydrogen production. J. Photochem. Photobiol. 216, 250–255 (2010).
Müller, B. R., Majoni, S., Memming, R. & Meissner, D. Particle size and surface chemistry in photoelectrochemical reactions at semiconductor particles. J. Phys. Chem. B 101, 2501–2507 (1997).
Katoh, R., Mural, M. & Furube, A. Electron–hole recombination in the bulk of a rutile TiO2 single crystal studied by sub-nanosecond transient absorption spectroscopy. Chem. Phys. Lett. 461, 238–241 (2008).
Shindler, K.-M. & Kunst, M. Charge-carrier dynamics in TiO2 powders. J. Phys. Chem. 94, 8222–8226 (1990).
Yamakata, A., Ishibashi, T-A. & Onishi, H. Time-resolved infrared absorption study of nine TiO2 photocatalysts. Chem. Phys. 339, 133–137 (2007).
Idriss, H. et al. A phenomenological study of the metal–oxide interface: The role of catalysis in hydrogen production from renewable resources. ChemSusChem 1, 905–910 (2008).
Hugon, A., Delannoy, L. & Louis, C. Supported gold catalysts for selective hydrogenation of 1,3-butadiene in the presence of an excess of alkenes. Gold Bulletin 41, 127–138 (2008).
Waterhouse, G. I. N. & Waterland, M. R. Opal and inverse opal photonic crystals: Fabrication and characterisation. Polyhedron 26, 356–368 (2007).
Acknowledgements
The authors would like to thank A. Goguet from Queen University of Belfast for discussions on gold particles. H.I. thanks Aberdeen City Council for a start-up grant to establish the Energy Futures Centre. The authors acknowledge the support of HEC Pakistan for the PhD scholarship to M.A.N. J.L. is grateful to grant CTQ2009-12520 (MICINN) and to ICREA Academia program (Generalitat de Catalunya), Spain.
Author information
Authors and Affiliations
Contributions
M.M. and M.A.N. conducted the photoreaction experiments, G.I.N.W. made the catalytic materials, J.L. produced the HRTEM and TEM images and provided the interpretation, H.I. conducted the XPS measurements, supervized M.M. and wrote the manuscript, J.B.M. supervised M.A.N., M.A.K. provided data interpretation for the photoreaction and R.H.F. provided data interpretation for the characterization of the materials.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 191 kb)
Rights and permissions
About this article
Cite this article
Murdoch, M., Waterhouse, G., Nadeem, M. et al. The effect of gold loading and particle size on photocatalytic hydrogen production from ethanol over Au/TiO2 nanoparticles. Nature Chem 3, 489–492 (2011). https://doi.org/10.1038/nchem.1048
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.1048
This article is cited by
-
Production of Biochar by Slow and Solar-Biomass Pyrolysis: Focus on the Output Configuration Assessment, Adaptability, and Barriers to Market Penetration
Arabian Journal for Science and Engineering (2024)
-
Preparation of enhanced visible light-responsive photocatalytic paper containing Ag/N-TiO2 aerogel for detoxification of environmental pollutants
Cellulose (2024)
-
Effect of Ag modification on TiO2 and melem/g-C3N4 composite on photocatalytic performances
Scientific Reports (2023)
-
Fiber-Optic Microfiber: Tracking Activity Enhancement and Suppression of Heterogeneous Photocatalysts
Advanced Fiber Materials (2023)
-
In-situ TiO2-x decoration of titanium carbide MXene for photo/sono-responsive antitumor theranostics
Journal of Nanobiotechnology (2022)