Abstract
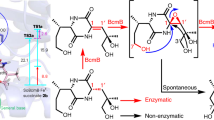
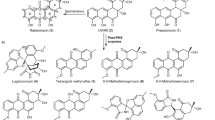
Oxidative cyclizations, exemplified by the biosynthetic assembly of the penicillin nucleus from a tripeptide precursor, are arguably the most synthetically powerful implementation of C–H activation reactions in nature. Here, we show that Rieske oxygenase-like enzymes mediate regio- and stereodivergent oxidative cyclizations to form 10- and 12-membered carbocyclic rings in the key steps of the biosynthesis of the antibiotics streptorubin B and metacycloprodigiosin, respectively. These reactions represent the first examples of oxidative carbocyclizations catalysed by non-haem iron-dependent oxidases and define a novel type of catalytic activity for Rieske enzymes. A better understanding of how these enzymes achieve such remarkable regio- and stereocontrol in the functionalization of unactivated hydrocarbon chains will greatly facilitate the development of selective man-made C–H activation catalysts.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Konomi, T. et al. Cell-free conversion of δ-(L-alpha-aminoadipyl)-L-cysteinyl-D-valine into an antibiotic with the properties of isopenicillin N in Cephalosporium acremonium. Biochem. J. 184, 427–430 (1979).
Elson, S. W. et al. Isolation of two novel intracellular β-lactams and a novel dioxygenase cyclizing enzyme from Streptomyces clavuligerus. J. Chem. Soc., Chem. Commun. 1736–1738 (1987).
Seto, H. et al. Studies on the biosynthesis of fosfomycin. 2. Conversion of 2-hydroxypropyl-phosphonic acid to fosfomycin by blocked mutants of Streptomyces wedmorensis. J. Antibiot. 44, 1286–1288 (1991).
Hammerschmidt, F. Biosynthesis of natural products with a P–C bond. Part 8: on the origin of the oxirane oxygen atom of fosfomycin in Streptomyces fradiae. J. Chem. Soc. Perkin Trans. 1 1993–1996 (1991).
Zerbe, K. et al. An oxidative phenol coupling reaction catalyzed by OxyB, a cytochrome P450 from the vancomycin-producing microorganism. Angew. Chem. Int. Ed. 43, 6709–6713 (2004).
Hollander, I. J., Shen, Y.-Q., Heim, J., Demain, A. L. & Wolfe, S. A pure enzyme catalyzing penicillin biosynthesis. Science 224, 610–612 (1984).
Liu, P. et al. Protein purification and function assignment of the epoxidase catalyzing the formation of fosfomycin. J. Am. Chem. Soc. 123, 4619–4620 (2001).
Roach, P. L. et al. Crystal structure of isopenicillin N synthase is the first from a new structural family of enzymes. Nature 375, 700–704 (1995).
Zhang, Z. et al. Structural origins of the selectivity of the trifunctional oxygenase clavaminic acid synthase. Nature Struct. Biol. 7, 127–133 (2000).
Higgins, L. J., Yan, F., Liu, P., Liu, H.-W. & Drennan, C. L. Structural insight into antibiotic fosfomycin biosynthesis by a mononuclear iron enzyme. Nature 437, 838–844 (2005).
Zerbe, K. et al. Crystal structure of OxyB, a cytochrome P450 implicated in an oxidative phenol coupling reaction during vancomycin biosynthesis. J. Biol. Chem. 277, 47476–47485 (2002).
Howard-Jones A. R. & Walsh, C. T. Staurosporine and rebeccamycin aglycones are assembled by the oxidative action of StaP, StaC and RebC on chromopyrrolic acid. J. Am. Chem. Soc. 128, 12289–12298 (2006).
Winkler A. et al. A concerted mechanism for berberine bridge enzyme. Nature Chem. Biol. 4, 739–741 (2008).
Roach, P. L. et al. Structure of isopenicillin N synthase complexed with substrate and the mechanism of penicillin formation. Nature 387, 827–830 (1997).
Burzlaff, N. I. et al. The reaction cycle of isopenicillin N synthase observed by X-ray diffraction. Nature 401, 721–724 (1999).
Price, J. C., Barr, E. W., Tirupati, B., Bollinger, J. M. Jr & Krebs, C. The first direct characterization of a high-valent iron intermediate in the reaction of an α-ketoglutarate-dependent dioxygenase: a high-spin FeIV complex in taurine/α-ketoglutarate dioxygenase (TauD) from Escherichia coli. Biochemistry 42, 7497–7508 (2003).
Mirica, L. M., McCusker, K. P., Munos, J. W., Liu, H.-W. & Klinman, J. P. 18O kinetic isotope effects in non-heme iron enzymes: probing the nature of Fe/O2 intermediates J. Am. Chem. Soc. 130, 8122–8123 (2008).
Wasserman, H. H., Shaw, C. K., Sykes, R. J. & Cushley, R. J. Biosynthesis of prodigiosin. III. Carbon-13 Fourier transform NMR. X. Biosynthesis of metacycloprodigiosin and undecylprodigiosin. Tetrahedron Lett. 2787–2790 (1974).
Wasserman, H. H., Rodgers, G. C. & Keith, D. D. Metacycloprodigiosin, a tripyrrole pigment from Streptomyces longisporus ruber. J. Am. Chem. Soc. 91, 1263–1264 (1969).
Wasserman, H. H., Rodgers, G. C. & Keith, D. D. Structure and synthesis of undecylprodigiosin. Prodigiosin analog from Streptomyces. Chem. Commun. 825–826 (1966).
Laatsch, H., Kellner, M. & Weyland, H. Butyl-meta-cycloheptylprodiginine — a revision of the structure of the former ortho-isomer. J. Antibiot. 44, 187–191 (1991).
Kawasaki, T., Sakurai, F. & Hayakawa, Y. A prodigiosin from the roseophilin producer Streptomyces griseoviridis. J. Nat. Prod. 71, 1265–1267 (2008).
Kayakawa, Y., Kawakami, K., Seto, H. & Furihata, K. Structure of a new antibiotic, roseophilin. Tetrahedron Lett. 33, 2701–2704 (1992).
Nguyen, M. et al. Small molecule obatoclax (GX15-070) antagonizes MCL-1 and overcomes MCL-1-mediated resistance to apoptosis. Proc. Natl Acad. Sci. USA 104, 19512–19517 (2007).
Mo, S. J. et al. Elucidation of the Streptomyces coelicolor pathway to 2-undecylpyrrole, a key intermediate in undecylprodiginine and streptorubin B biosynthesis. Chem. Biol. 15, 137–148 (2008).
Stanley, A. E., Walton, L. J., Kourdi-Zerikly, M., Corre, C. & Challis, G. L. Elucidation of the Streptomyces coelicolor pathway to 4-methoxy-2,2′-bipyrrole-5-carboxaldehyde, an intermediate in prodiginine biosynthesis. Chem. Commun. 3981–3983 (2006).
Haynes, S. W., Sydor, P. K., Stanley, A. E., Song, L. & Challis, G. L. Role and substrate specificity of the Streptomyces coelicolor RedH enzyme in undecylprodiginine biosynthesis. Chem. Commun. 1865–1867 (2008).
Cerdeno, A. M., Bibb M. J. & Challis, G. L. Analysis of the prodiginine biosynthesis gene cluster of Streptomyces coelicolor A3(2): new mechanisms for chain initiation and termination in modular multienzymes. Chem. Biol. 8, 817–829 (2001).
Kauppi, B. et al. Structure of an aromatic-ring-hydroxylating dioxygenase-naphthalene 1,2-dioxygenase. Structure 6, 571–586 (1998).
Gibson, D. T. & Parales R. E. Aromatic hydrocarbon dioxygenases in environmental biotechnology. Curr. Opin. Biotechnol. 11, 236–243 (2000).
Lee, J., Simurdiak, M. & Zhao, H. Reconstitution and characterization of aminopyrrolnitrin oxygenase, a Rieske N-oxygenase that catalyzes unusual arylamine oxidation. J. Biol. Chem. 280, 36719–36727 (2005).
Ferraro D. J., Gakhar L. & Ramaswamy S. Rieske business: structure–function of Rieske non-heme oxygenases. Biochem. Biophys. Res. Commun. 338, 175–190 (2005).
Gust, B., Challis, G. L., Fowler, K., Kieser T., & Chater K. F. PCR-targeted Streptomyces gene replacement identifies a protein domain needed for biosynthesis of the sesquiterpene soil odor geosmin. Proc. Natl Acad. Sci. USA 100, 1541–1546 (2003).
Hu, D. X., Clift, M. D., Lazarski, K. E. & Thomson R. J. Enantioselective total synthesis and confirmation of the absolute and relative stereochemistry of streptorubin B. J. Am. Chem. Soc. 133, 1799–1804 (2011).
Haynes, S. W., Sydor, P. K., Corre, C., Song, L. & Challis G. L. Stereochemical elucidation of streptorubin B. J. Am. Chem. Soc. 133, 1793–1798 (2011).
Kawasaki, T., Sakurai, F., Nagatsuka, S. & Hayakawa, Y. Prodigiosin biosynthesis gene cluster in the roseophilin producer Streptomyces griseoviridis. J. Antibiot. 62, 271–276 (2009).
Bugg, T. D. H. & Ramaswamy, S. Non-heme iron-dependent dioxygenases: unraveling catalytic mechanisms for complex enzymatic oxidations. Curr. Opin. Chem. Biol. 12, 134–140 (2008).
Chakrabarty, S., Austin, R. N., Deng, D., Groves, J. T. & Lipscomb, J. D. Radical intermediates in monooxygenase reactions of Rieske dioxygenases. J. Am. Chem. Soc. 129, 3514–3515 (2007).
Chen, M. S. & White, M. C. A predictably selective aliphatic C–H oxidation reaction for complex molecule synthesis. Science 318, 783–787 (2007).
Stang, E. M. & White, M. C. Total synthesis and study of 6-deoxyerythronolide B by late-stage C–H oxidation. Nature Chem. 1, 547–551 (2009).
Acknowledgements
The authors acknowledge financial support from the University of Warwick, the National Institutes of Health (1R01GM77147-01A1), the Engineering and Physical Sciences Research Council and the European Union (contract no. 005224). The authors also thank R. Thomson for kindly providing the synthetic sample of the carbocyclic derivative of 2-undecylpyrrole. The assistance of D. Oves-Costales with the chemical conversion of desmethylundecylprodigiosin to undecylprodigiosin is gratefully acknowledged.
Author information
Authors and Affiliations
Contributions
P.K.S., S.M.B., O.M.O., F.B.G., S.W.H, C.C., L.S. and G.L.C. designed the research. P.K.S., S.M.B., O.M.O., F.B.G., S.W.H, C.C. and L.S. performed the research. P.K.S., S.M.B., O.M.O., F.B.G., S.W.H, C.C., L.S. and G.L.C. interpreted the data. G.L.C., P.K.S. and S.M.B. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 804 kb)
Rights and permissions
About this article
Cite this article
Sydor, P., Barry, S., Odulate, O. et al. Regio- and stereodivergent antibiotic oxidative carbocyclizations catalysed by Rieske oxygenase-like enzymes. Nature Chem 3, 388–392 (2011). https://doi.org/10.1038/nchem.1024
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.1024
This article is cited by
-
Prodigiosin R3, a new multicyclic prodigiosin formed by prodigiosin cyclization genes in the roseophilin producer
The Journal of Antibiotics (2023)
-
AvmM catalyses macrocyclization through dehydration/Michael-type addition in alchivemycin A biosynthesis
Nature Communications (2022)
-
Structures, biosynthesis, and bioactivities of prodiginine natural products
Applied Microbiology and Biotechnology (2022)
-
The complete genomic sequence of Streptomyces spectabilis NRRL-2792 and identification of secondary metabolite biosynthetic gene clusters
Journal of Industrial Microbiology and Biotechnology (2019)
-
A Rieske oxygenase/epoxide hydrolase-catalysed reaction cascade creates oxygen heterocycles in mupirocin biosynthesis
Nature Catalysis (2018)