Abstract
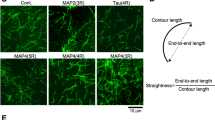
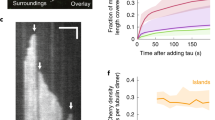
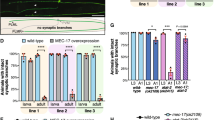
Regulated increase in the formation of microtubule arrays is thought to be important for axonal growth. Collapsin response mediator protein-2 (CRMP-2) is a mammalian homologue of UNC-33, mutations in which result in abnormal axon termination. We recently demonstrated that CRMP-2 is critical for axonal differentiation. Here, we identify two activities of CRMP-2: tubulin-heterodimer binding and the promotion of microtubule assembly. CRMP-2 bound tubulin dimers with higher affinity than it bound microtubules. Association of CRMP-2 with microtubules was enhanced by tubulin polymerization in the presence of CRMP-2. The binding property of CRMP-2 with tubulin was apparently distinct from that of Tau, which preferentially bound microtubules. In neurons, overexpression of CRMP-2 promoted axonal growth and branching. A mutant of CRMP-2, lacking the region responsible for microtubule assembly, inhibited axonal growth and branching in a dominant-negative manner. Taken together, our results suggest that CRMP-2 regulates axonal growth and branching as a partner of the tubulin heterodimer, in a different fashion from traditional MAPs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Craig, A. M. & Banker, G. Neuronal polarity. Annu. Rev. Neurosci. 17, 267–310 (1994).
Goslin, K. & Banker, G. Experimental observations on the development of polarity by hippocampal neurons in culture. J. Cell Biol. 108, 1507–1516 (1989).
Esch, T., Lemmon, V. & Banker, G. Local presentation of substrate molecules directs axon specification by cultured hippocampal neurons. J. Neurosci. 19, 6417–6426 (1999).
Baas, P. W. Microtubules and axonal growth. Curr. Opin. Cell Biol. 9, 29–36 (1997).
Baas, P. W. Microtubules and neuronal polarity: lessons from mitosis. Neuron 22, 23–31 (1999).
Hirokawa, N. Microtubule organization and dynamics dependent on microtubule-associated proteins. Curr. Opin. Cell Biol. 6, 74–81 (1994).
Drechsel, D. N. et al. Modulation of the dynamic instability of tubulin assembly by the microtubule-associated protein tau. Mol. Biol. Cell 3, 1141–1154 (1992).
Garcia, M. L. & Cleveland, D. W. Going new places using an old MAP: tau, microtubules and human neurodegenerative disease. Curr. Opin. Cell Biol. 13, 41–48 (2001).
Goshima, Y. et al. Collapsin-induced growth-cone collapse mediated by an intracellular protein related to UNC-33. Nature 376, 509–514 (1995).
Minturn, J. E. et al. TOAD-64, a gene expressed early in neuronal differentiation in the rat, is related to unc-33, a C. elegans gene involved in axon outgrowth. J. Neurosci. 15, 6757–6766 (1995).
Gaetano, C., Matsuo, T. & Thiele, C. J. Identification and characterization of a retinoic acid-regulated human homologue of the unc-33-like phosphoprotein gene (hUlip) from neuroblastoma cells. J. Biol. Chem. 272, 12195–12201 (1997).
Byk, T., Ozon, S. & Sobel, A. The Ulip family phosphoproteins — common and specific properties. Eur. J. Biochem. 254, 14–24 (1998).
Inatome, R. et al. Identification of CRAM, a novel unc-33 gene family protein that associates with CRMP3 and protein-tyrosine kinase(s) in the developing rat brain. J. Biol. Chem. 275, 27291–27302 (2000).
Hedgecock, E. M. et al. Axonal guidance mutants of Caenorhabditis elegans identified by filling sensory neurons with fluorescein dyes. Dev. Biol. 111, 158–170 (1985).
Arimura, N. et al. Phosphorylation of collapsin response mediator protein-2 by rho-kinase. Evidence for two separate signaling pathways for growth-cone collapse. J. Biol. Chem. 275, 23973–23980 (2000).
Inagaki, N. et al. CRMP-2 induces axons in cultured hippocampal neurons. Nature Neurosci. 4, 781–782 (2001).
Wang, L. H. & Strittmatter, S. M. Brain CRMP forms heterotetramers similar to liver dihydropyrimidinase. J. Neurochem. 69, 2261–2269 (1997).
Detrich, H. W. 3rd & Williams, R. C. Reversible dissociation of the αβ dimer of tubulin from bovine brain. Biochemistry 17, 3900–3907 (1978).
Luduena, R. F., Shooter, E. M. & Wilson, L. Structure of the tubulin dimer. J. Biol. Chem. 252, 7006–7014 (1977).
Horio, T. & Hotani, H. Visualization of the dynamic instability of individual microtubules by dark-field microscopy. Nature 321, 605–607 (1986).
Itoh, T. J. et al. Phosphorylation states of microtubule-associated protein 2 (MAP2) determine the regulatory role of MAP2 in microtubule dynamics. Biochemistry 36, 12574–12582 (1997).
Itoh, T. J. & Hotani, H. Microtubule-stabilizing activity of microtubule-associated proteins (MAPs) is due to increase in frequency of rescue in dynamic instability: shortening length decreases with binding of MAPs onto microtubules. Cell Struct. Funct. 19, 279–290 (1994).
Gustke, N. et al. Domains of tau protein and interactions with microtubules. Biochemistry 33, 9511–9522 (1994).
Pedrotti, B. & Islam, K. Purified native microtubule associated protein MAP1A: kinetics of microtubule assembly and MAP1A/tubulin stoichiometry. Biochemistry 33, 12463–12470 (1994).
Gu, Y. & Ihara, Y. Evidence that collapsin response mediator protein-2 is involved in the dynamics of microtubules. J. Biol. Chem. 275, 17917–17920 (2000).
Jalink, K. et al. Inhibition of lysophosphatidate- and thrombin-induced neurite retraction and neuronal cell rounding by ADP ribosylation of the small GTP-binding protein Rho. J. Cell Biol. 126, 801–810 (1994).
Leger, J. G., Brandt, R. & Lee, G. Identification of tau protein regions required for process formation in PC12 cells. J. Cell Sci. 107, 3403–3412 (1994).
Dotti, C. G., Sullivan, C. A. & Banker, G. A. The establishment of polarity by hippocampal neurons in culture. J. Neurosci. 8, 1454–1468 (1988).
Brown, A. et al. Composite microtubules of the axon: quantitative analysis of tyrosinated and acetylated tubulin along individual axonal microtubules. J. Cell Sci. 104, 339–352 (1993).
Black, M. M. et al. Tau is enriched on dynamic microtubules in the distal region of growing axons. J. Neurosci. 16, 3601–3619 (1996).
Yu, W., Ahmad, F. J. & Baas, P. W. Microtubule fragmentation and partitioning in the axon during collateral branch formation. J. Neurosci. 14, 5872–5884 (1994).
Gallo, G. & Letourneau, P. C. Different contributions of microtubule dynamics and transport to the growth of axons and collateral sprouts. J. Neurosci. 19, 3860–3873 (1999).
Yu, W. & Baas, P. W. Changes in microtubule number and length during axon differentiation. J. Neurosci. 14, 2818–2829 (1994).
Ahmad, F. J. & Baas, P. W. Microtubules released from the neuronal centrosome are transported into the axon. J. Cell Sci. 108, 2761–2769 (1995).
Yu, W., Schwei, M. J. & Baas, P. W. Microtubule transport and assembly during axon growth. J. Cell Biol. 133, 151–157 (1996).
Rochlin, M. W., Wickline, K. M. & Bridgman, P. C. Microtubule stability decreases axon elongation but not axoplasm production. J. Neurosci. 16, 3236–3246 (1996).
Nixon, R. A. The slow axonal transport of cytoskeletal proteins. Curr. Opin. Cell Biol. 10, 87–92 (1998).
Okabe, S. & Hirokawa, N. Axonal transport. Curr. Opin. Cell Biol. 1, 91–97 (1989).
Shah, J. V. & Cleveland, D. W. Slow axonal transport: fast motors in the slow lane. Curr. Opin. Cell Biol. 14, 58–62 (2002).
Brown, A., Slaughter, T. & Black, M. M. Newly assembled microtubules are concentrated in the proximal and distal regions of growing axons. J. Cell Biol. 119, 867–882 (1992).
Yu, W. & Baas, P. W. The growth of the axon is not dependent upon net microtubule assembly at its distal tip. J. Neurosci. 15, 6827–6833 (1995).
Kanai, Y. & Hirokawa, N. Sorting mechanisms of tau and MAP2 in neurons: suppressed axonal transit of MAP2 and locally regulated microtubule binding. Neuron 14, 421–432 (1995).
Gu, Y., Hamajima, N. & Ihara, Y. Neurofibrillary tangle-associated collapsin response mediator protein-2 (CRMP-2) is highly phosphorylated on Thr-509, Ser-518, and Ser-522. Biochemistry 39, 4267–4275 (2000).
Acknowledgements
We thank Y. Gu, Y. Ihara, Y. Kanai, N. Hirokawa, N. J. Cowan, E. Mekada, T. Kato and M. Nakafuku for kind gifts of materials. We also thank M. Amano, M. Fukata, S. Taya, Y. Kawano (Nagoya University) and H. Qadota (Nara Institute of Science and Technology) for helpful discussion and for preparing some materials, and T. Ishii and M. Yoshizaki for secretarial and technical assistance. This research was supported in part by Grants-in-Aid for Scientific Research from the Ministry of Education, Science, Sports and Culture of Japan, the Japan Society for the Promotion of Science Research for the Future and the Human Frontier Science Program. F.Y. is a research fellow of the Japan Society for the Promotion of Science.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary figure
Figure S1 Direct interaction between CRMP-2 and tubulin heterodimers. (PDF 193 kb)
Rights and permissions
About this article
Cite this article
Fukata, Y., Itoh, T., Kimura, T. et al. CRMP-2 binds to tubulin heterodimers to promote microtubule assembly. Nat Cell Biol 4, 583–591 (2002). https://doi.org/10.1038/ncb825
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb825
This article is cited by
-
Advances in Understanding the Molecular Mechanisms of Neuronal Polarity
Molecular Neurobiology (2023)
-
Genome-wide translation control analysis of developing human neurons
Molecular Brain (2022)
-
Phosphorylation of CRMP2 by Cdk5 Negatively Regulates the Surface Delivery and Synaptic Function of AMPA Receptors
Molecular Neurobiology (2022)
-
DPYSL2 as potential diagnostic and prognostic biomarker linked to immune infiltration in lung adenocarcinoma
World Journal of Surgical Oncology (2021)
-
Inhibition of RhoA Activity Does Not Rescue Synaptic Development Abnormalities and Long-Term Cognitive Impairment After Sevoflurane Exposure
Neurochemical Research (2021)