Abstract
Adult stem cells provide a renewable source of differentiated cells for a wide variety of tissues and generally give rise to multiple cell types. Basic principles of stem cell organization and regulation underlying this behaviour are emerging. Local niche signals maintain stem cells, while different sets of signals act outside the niche to diversify initially equivalent stem cell progeny. Here we show that Drosophila ovarian follicle stem cells (FSCs) produced two distinct cell types directly. This cell fate choice was determined by the anterior–posterior position of an FSC and by the magnitude of spatially graded Wnt pathway activity. These findings reveal a paradigm of immediate diversification of stem cell derivatives according to stem cell position within a larger population, guided by a graded niche signal. We also found that FSCs strongly resemble mammalian intestinal stem cells in many aspects of their organization, including population asymmetry and dynamic heterogeneity.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Clevers, H. The intestinal crypt, a prototype stem cell compartment. Cell 154, 274–284 (2013).
Losick, V. P., Morris, L. X., Fox, D. T. & Spradling, A. Drosophila stem cell niches: a decade of discovery suggests a unified view of stem cell regulation. Dev. Cell 21, 159–171 (2011).
Eliazer, S. & Buszczak, M. Finding a niche: studies from the Drosophila ovary. Stem Cell Res. Ther. 2, 45 (2011).
Margolis, J. & Spradling, A. Identification and behavior of epithelial stem cells in the Drosophila ovary. Development 121, 3797–3807 (1995).
Nystul, T. & Spradling, A. An epithelial niche in the Drosophila ovary undergoes long-range stem cell replacement. Cell Stem Cell 1, 277–285 (2007).
Nystul, T. & Spradling, A. Regulation of epithelial stem cell replacement and follicle formation in the Drosophila ovary. Genetics 184, 503–515 (2010).
Zhang, Y. & Kalderon, D. Hedgehog acts as a somatic stem cell factor in the Drosophila ovary. Nature 410, 599–604 (2001).
Decotto, E. & Spradling, A. C. The Drosophila ovarian and testis stem cell niches: similar somatic stem cells and signals. Dev. Cell 9, 501–510 (2005).
Kirilly, D., Wang, S. & Xie, T. Self-maintained escort cells form a germline stem cell differentiation niche. Development 138, 5087–5097 (2011).
Lee, T. & Luo, L. Mosaic analysis with a repressible cell marker (MARCM) for Drosophila neural development. Trends Neurosci. 24, 251–254 (2001); erratum 24, 385 (2001).
Kronen, M. R., Schoenfelder, K. P., Klein, A. M. & Nystul, T. G. Basolateral junction proteins regulate competition for the follicle stem cell niche in the Drosophila ovary. PLoS ONE 9, e101085 (2014).
Sahai-Hernandez, P. & Nystul, T. G. A dynamic population of stromal cells contributes to the follicle stem cell niche in the Drosophila ovary. Development 140, 4490–4498 (2013).
Blanpain, C. & Simons, B. D. Unravelling stem cell dynamics by lineage tracing. Nat. Rev. Mol. Cell Biol. 14, 489–502 (2013).
Simons, B. D. & Clevers, H. Strategies for homeostatic stem cell self-renewal in adult tissues. Cell 145, 851–862 (2011).
Chang, Y. C., Jang, A. C., Lin, C. H. & Montell, D. J. Castor is required for Hedgehog-dependent cell-fate specification and follicle stem cell maintenance in Drosophila oogenesis. Proc. Natl Acad. Sci. USA 110, E1734–E1742 (2013).
Hartman, T. R. et al. Novel tools for genetic manipulation of follicle stem cells in the Drosophila ovary reveal an integrin-dependent transition from quiescence to proliferation. Genetics 199, 935–957 (2015).
Song, X. et al. Bmp signals from niche cells directly repress transcription of a differentiation-promoting gene, bag of marbles, in germline stem cells in the Drosophila ovary. Development 131, 1353–1364 (2004).
Buszczak, M. et al. The Carnegie protein trap library: a versatile tool for Drosophila developmental studies. Genetics 175, 1505–1531 (2007).
Vied, C., Reilein, A., Field, N. S. & Kalderon, D. Regulation of stem cells by intersecting gradients of long-range niche signals. Dev. Cell 23, 836–848 (2012).
Forbes, A. J., Spradling, A. C., Ingham, P. W. & Lin, H. The role of segment polarity genes during early oogenesis in Drosophila. Development 122, 3283–3294 (1996).
Vied, C. & Kalderon, D. Hedgehog-stimulated stem cells depend on non-canonical activity of the Notch co-activator Mastermind. Development 136, 2177–2186 (2009).
Bach, E. A. et al. GFP reporters detect the activation of the Drosophila JAK/STAT pathway in vivo. Gene Expr. Patterns 7, 323–331 (2007).
Wang, X. & Page-McCaw, A. A matrix metalloproteinase mediates long-distance attenuation of stem cell proliferation. J. Cell Biol. 206, 923–936 (2014).
Song, X. & Xie, T. Wingless signaling regulates the maintenance of ovarian somatic stem cells in Drosophila. Development 130, 3259–3268 (2003).
Huang, J. & Kalderon, D. Coupling of Hedgehog and Hippo pathways promotes stem cell maintenance by stimulating proliferation. J. Cell Biol. 205, 325–338 (2014).
Wang, Z. A., Huang, J. & Kalderon, D. Drosophila follicle stem cells are regulated by proliferation and niche adhesion as well as mitochondria and ROS. Nat. Commun. 3, 769 (2012).
Wang, Z. A. & Kalderon, D. Cyclin E-dependent protein kinase activity regulates niche retention of Drosophila ovarian follicle stem cells. Proc. Natl Acad. Sci. USA 106, 21701–21706 (2009).
Krieger, T. & Simons, B. D. Dynamic stem cell heterogeneity. Development 142, 1396–1406 (2015).
Ritsma, L. et al. Intestinal crypt homeostasis revealed at single-stem-cell level by in vivo live imaging. Nature 507, 362–365 (2014).
Basak, O. et al. Mapping early fate determination in Lgr5+ crypt stem cells using a novel Ki67-RFP allele. EMBO J. 33, 2057–2068 (2014).
Buczacki, S. J. et al. Intestinal label-retaining cells are secretory precursors expressing Lgr5. Nature 495, 65–69 (2013).
Schuijers, J. et al. Ascl2 acts as an R-spondin/Wnt-responsive switch to control stemness in intestinal crypts. Cell Stem Cell 16, 158–170 (2015).
Stamataki, D. et al. Delta1 expression, cell cycle exit, and commitment to a specific secretory fate coincide within a few hours in the mouse intestinal stem cell system. PLoS ONE 6, e24484 (2011).
Kambadur, R. et al. Regulation of POU genes by castor and hunchback establishes layered compartments in the Drosophila CNS. Genes Dev. 12, 246–260 (1998).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012).
Prasad, M., Jang, A. C., Starz-Gaiano, M., Melani, M. & Montell, D. J. A protocol for culturing Drosophila melanogaster stage 9 egg chambers for live imaging. Nat. Protoc. 2, 2467–2473 (2007).
Olson, E. R. et al. Yan, an ETS-domain transcription factor, negatively modulates the Wingless pathway in the Drosophila eye. EMBO Rep. 12, 1047–1054 (2011).
Griffin, R. et al. The twin spot generator for differential Drosophila lineage analysis. Nat. Methods 6, 600–602 (2009).
Acknowledgements
We thank Y. Veras, R. Toueg and N. Field for technical assistance, D. Rabinowitz and H. Bussenmaker for help with statistical analyses, W. Odenwald (National Institute of Neurological Disorders and Stroke, USA), R. DasGupta (Genome Institute of Singapore, Singapore), E. Bach (New York University School of Medicine, USA), the Developmental Studies and the Bloomington Stock Center for antibodies and fly stocks, and T. Hazelrigg, M. Shen, M. Crist and J. Little for comments on the manuscript. This work was supported by the National Institutes of Health (RO1 GM079351 to D.K.); D.M. was supported in part by an NIH training grant.
Author information
Authors and Affiliations
Contributions
Conceptualization, A.R. and D.K.; methodology, A.R., E.C., N.T., G.V.-N. and D.K.; formal analysis, A.R., D.M. and D.K.; investigation, A.R., D.M., K.S.P., A.B. and S.F.; writing original draft, D.K.; writing-review and editing, A.R., D.M. and D.K.; visualization, A.R. and D.K.; funding acquisition, D.K.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
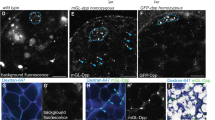
Supplementary Figure 1 Multicolour labeling method, imaging and scoring.
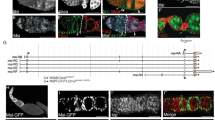
(a) Second chromosome genotype (top) of flies used for lineage marking, showing tub-lacZ (lacZ), ubi-GFP (GFP) and ubi-RFP (RFP) transgenes and FRT 40A and FRT 42B recombination targets either side of the centromere (white oval). Heat-shock (HS) induction of a hs-flp transgene on the X-chromosome can induce recombination independently at either pair of FRTs, making one or both (2L and 2R) chromosome arms homozygous in daughter cells, thereby eliminating one or more of the marker genes (one possible outcome for one daughter cell is shown). The resulting daughter cell is designated ‘BR’ to indicate the presence of just two colours (Blue and Red) compared to the three colours (BGR) present for parental cells after staining (B- Blue lacZ, G-Green GFP, R- Red, RFP). (b) The nine possible genotypes that can be produced by FRT-mediated recombination are indicated by the combination of 2L genotypes (labeled vertically) and 2R genotypes (labeled horizontally: ‘ + ’ indicates the absence of the RFP transgene). The resulting diploid genotypes are displayed within the nine large boxes; each vertical column of small coloured squares represents one chromosome that may include lacZ (Blue), GFP (Green) or RFP (Red) transgenes or not (white). Two copies of ubi-RFP could not reliably be distinguished phenotypically from one, so there are only six distinguishable phenotypes (indicated by the combination of Blue (B), Green (G) and Red (R) colours present). Only the three RFP-negative phenotypes on the right (BG, B and G) are produced by just a single genotype. Accordingly, these RFP-negative phenotypes are expected to be the least frequent. In the 9d AHS (after heat-shock) experiment RFP-negative FSC lineages were indeed present at a frequency (52/177) roughly half that of RFP-positive FSC lineages (125/177). (c) Illustration of one possible distribution of FSC genotypes in a germarium. Each FSC is represented by a coloured circle within one of the nine boxes representing the nine possible genotypes. Note that two (or more) FSCs may acquire identical genotypes (BGR, top left) or may share the same RFP-positive phenotype despite having different genotypes (for example, GR FSCs in the left and middle columns). Hence, in this germarium, seven FSCs exhibit just four distinct phenotypes. (d–n) Images of the same ovariole shown in Fig. 1c, d to illustrate (d–h) how single channel images are used to assign transgenes present and (i–n) how a complete series of z-sections is used to assign FSC positions. (d–h) Images show (d) all channels, (e) Fas3 antibody staining (white), (f) β-galactosidase antibody staining (blue, ‘B’), (g) GFP (green, ‘G’) and (h) RFP (red, ‘R’) for a central z-section. The red channel includes some bleed-through from Fas3 staining (from Alexa 546) but the RFP signal is distinguished as nuclear, while Fas3 staining is at the plasma membrane (germline nuclei also stain in one or more channels but are easily distinguished by their size and location). (i–n) Successive z-sections encompassing the germarium (one section between j and k is omitted because it shows only FSCs seen in adjacent sections) with each FSC labeled according to colour (BGR, GR, B, BR). (l) The B FSC seen in (k) and the two lower GR FSCs seen in (m) are also seen in this section but are not labeled, so that each FSC is labeled only once in (i–n). Yellow arrows mark the borders of Fas3 expression (pink in composites). Brackets or arrows marking FSCs are labeled according to layers, with layer 1 (white) adjacent to Fas3, layer 2 (cyan) immediately anterior (left) and layer 3 (yellow) one cell further anterior (left). Fourteen FSCs are shown (1 B, 2 BR, 8 GR, 3 BGR), with one (GR*) in (n) in the process of mitosis. Note that there is a single candidate B FSC (as stated for Fig. 1c) and also note that the BGR FSCs are not seen in central z-sections (the 60 ° sectors shown in Fig. 1e). Also, there are no G or BG FSCs even though G and BG FCs are present (f–h and Fig. 1d), indicating that G and BG FSCs were lost between day 5 and day 9. Scale bar of 20 μm applies to all panels (all are same magnification).
Supplementary Figure 2 Decline in number of FSC lineages over time indicates population asymmetry.
(a–c) Percentage of ovarioles (among 50 scored) with the indicated number of distinct clones 9d AHS defined by inclusion of (a) both FSCs and FCs (‘FSC clones’), (b) FSCs (with or without matching FCs) and (c) FCs (with or without FSCs), representing all active FSCs present at 5d. To the right of each observed distribution is the expected distribution of distinct colours predicted from a model (in blue) for the number of FSCs (5, 6 and 10, respectively) that best fits the adjacent experimental distribution (see Supplementary Note). (d–j) Examples of (f,g,j) ovarioles and (d,e,h,i) their germaria at higher magnification from multi-colour FSC lineage experiments examined at 9 days AHS. (d–g) Illustration of an FSC-only clone (such clones are included in (b) but not (a)). The same (d,e) germarium and (f,g) ovariole with (d,f) all colours present or (e,g) only the green channel (to clearly distinguish B from BG cells), showing B FSCs with no matching FC. (d,e) have seven z-sections combined to show all FSCs. (f,g) Vertical white lines separate different z-sections (indicated top right) for different regions of the ovariole (full original images for each z-section in Supplementary Fig. 9). (h–j) Illustration of an FC-only clone (such clones are included in (c) but not (a)). (h,i) Two combinations of z-sections (z1–3 and z5–8 to show all FSCs) of one germarium and (j) egg chambers from the same ovariole, showing BG FC patches with no BG FSC. Scale bars are 20 μm. (k–n) Percentage of all B, G and BG multicolour or GFP+ MARCM FSC clones that contain a given (k,l,n) number or (m) range of FSCs when analyzed at the stated times after clone induction. The total number of biologically independent clones analyzed was (k) n = 64, (l) n = 83, (m) n = 83 (9d), n = 43 (22d) and (n) n = 20.
Supplementary Figure 3 FSC locations from multicolor lineages induced by a single heat-shock.
(a–h) Examples of (a,c,e,g) germaria (at higher magnification) and (b, d, f, h) their associated ovarioles from multi-colour FSC lineage experiments examined 12 days after a single heat-shock. (a,b) The only candidate FSC of the BR lineage is in layer 1 (‘BR’, white arrow). (c,d) The only candidate FSC of the BGR lineage is in layer 2 (‘BGR’, cyan arrow). (e,f) The only candidate FSC of the BR lineage is in layer 3 (‘BR’, yellow arrow). (g,h) The only candidate FSC of the BG lineage is in layer 1 and is at the bottom surface of the germarium, not in a mid-section (‘BG’, white arrow). The border of Fas3 staining (pink) is outlined by a white dotted line in (a,c,e,g). (a,c) have two z-sections combined and (e) has four z-sections superimposed. (h) Vertical white line separates different z-sections (indicated top right) for different regions of the ovariole (full original images for each z-section in Supplementary Fig. 9). (b,d,h) Diagonal white lines indicate an edge of the original image (shown in Supplementary Fig. 9). Scale bar, 20 μm in (a,c,e,g) and 50 μm in (b,d,f,h).
Supplementary Figure 4 Quantitation of Radial Cell Movements in the FSC region by Live Imaging.
(a) Graphical representation of radial cell movements for the germarium shown in Supplementary Video 6. Positions of cell bodies at each time point were placed around a circle representing a germarial cross-section in the FSC region. Each circle was subdivided into 12 sectors and 9 z-sections (red lines) to place the cells accurately based on their xyz coordinates at each imaging time-point, generally at 15 min intervals. Each GFP-marked cell nucleus could be tracked without ambiguity and is artificially coloured. The whole germarium sometimes moved, including rotations. The critical parameter is therefore the movement of one cell nucleus relative to another. Cell pairs continually switched between periods of moving towards one another and moving apart. (b) For each pair of cells (A–B, A–C etc.) we estimated and tabulated relative movement as the fractions of a sector (1/12 of the circumference) by which two cells separated (positive values in blue) or approached (negative values in pink) over the time interval during which they maintained their approach or separation. The minimum resolvable distance was 0.25 of a sector, so distances were estimated as multiples of quarter sectors. When it was too difficult to judge whether the relationship of cell pairs had changed, the movement was scored as 0. The proportion of time intervals during which cell pairs altered their radial separation was summed over the entire movie (only the first 4 h of the full 7 h movie are displayed as circles and tabulated here) as ‘% time moving’. The average rate at which each cell pair altered their separation during periods of movement was calculated as the percentage of the circumference moved per hour (‘% circ. moved/h’). The overall averages for 34 pairs of cells analyzed in four full movies are given in the two bottom cells. All 34 cell pairs exhibited continual back and forth movements.
Supplementary Figure 5 Anterior movement of FSCs into EC territory.
(a–c) Three time-stamped series of live imaging frames up to (a) 6 h 45 min, (b) 3 h 45 min and (c) 3 h 37 min from germaria where one marked cell (white arrow) moves anteriorly (left) from the FSC region to EC territory. In (c) a second FSC (purple arrow) moves a shorter distance anteriorly. All FSCs marked by arrows in (a–c) moved radially relative to each other. Panel a frames are from Supplementary Video 4, panel b from Supplementary Video 5, panel c from Supplementary Video 6. We observed four clear examples of large anterior movements, likely representing FSCs becoming ECs, in a total of about 80 recorded germaria, each containing an average of four labeled FSCs, viewed on average for about half a cycle (6 h). We deduced in our twin-spot analyses that approximately one EC is produced per cycle from the whole FSC population. We would therefore expect an EC to be produced from a quarter of the FSC population (4 labeled FSCs) viewed over half a cycle roughly once in every eight videos. Large anterior movements were observed in roughly 1/20 of videos (4/80). The frequency of these movements is consistent with FSCs becoming ECs but suggests that at least as many ECs were also produced as a result of smaller anterior movements of FSCs in the same set of videos. Scale bars are 20 μm.
Supplementary Figure 6 FSC heterogeneity along the A/P axis.
(a–f) Single cells labeled with surface CD8-GFP in Fas3 (red)-stained germaria. (a–c) FSCs in a section from the top or bottom third of the z-stacks, showing processes extending bilaterally from (a) layer 1, (b) layer 2 or (c) layer 3 FSCs. (d–g) ECs have (d) short processes in anterior regions but (e,f) longer processes contacting stage 2a cysts if in region 2, closer to the location of FSCs. (g–i) 109-30 GAL4 enhancer trap stained for the products of UAS-GFP (green), PZ1444-lacZ (red) and Fas3 (blue), showing 109-30-GAL4 expression in FSCs and FCs (similar to the pattern of Castor expression but less uniform). (g) and (h) are the same image with or without the blue channel. (i) is the projection of four z-sections, spanning 9 μm. (j–l) C587-GAL4 enhancer trap expressing UAS-CD8-RFP (red in j, gray-scale in l) and stained for expression of PZ1444-lacZ (green in j, gray-scale in k) and Fas3 (blue) showed strong C587-GAL4 expression in ECs and weaker expression in FSCs (similar to the pattern of PZ144-lacZ expression but extending weakly also into FCs). (m–o) ptc-lacZ expression in green (m) or gray-scale (n) from the same germarium and (o) in green in another example is high in ECs, FSCs and the earliest FCs. Arrows indicate Fas3 staining border (green), FSCs in layer 1 (white), layer 2 (cyan) and layer 3 (yellow), and early FCs (pink). All scale bars are 20 μm.
Supplementary Figure 7 Twin-spot method.
Recombination can occur at either FRT40A or FRT42B or both followed by a variety of possible segregation patterns. In the example shown, the daughter pairs are B and GR. All possible daughter pairs are listed but we did not score BG/BGR pairs because the BGR daughter cannot be distinguished from the many cells not undergoing any recombination.
Supplementary Figure 8 Early (6d) effects of altered Wnt pathway activity on FSC behaviour.
(a–c) Increased Wnt pathway activity due to axn or apc mutations (a) decreased the proportion of FSC clones associated with FCs, (b) increased the average number of labeled ECs per germarium, and (c) decreased the proportion of FSCs in layer 1 in favor of more anterior positions. These phenotypes at 6d were generally less pronounced than at 12d (Fig. 8). (e–g) Loss of Wnt pathway activity (e) increased the proportion of FSC clones associated with FCs and reduced the percentage of ovarioles with labeled ECs, (f) reduced the average number of labeled ECs per germarium and (g) increased the proportion of FSCs in layer 1 versus layers 2 and 3. (d) At 12d AHS increased Wnt pathway activity due to axn or apc mutations decreased the percentage of FSC clones associated with labeled FCs and increased the percentage of ovarioles with labeled ECs. (h,i) The fraction of MARCM-labeled FSCs labeled by EdU at 6d (h) was decreased significantly for layer 1 FSCs and the total FSC population by increased Wnt pathway activity and (i) was slightly but not significantly reduced in each layer by loss of Wnt pathway activity. In all cases there is still no significant EdU incorporation into ECs (not shown). Error bars show SEM for (a,b) n = 68 (WT), n = 65 (axnSO), n = 67 (axnE77) and n = 65 (apc1apc2) biologically independent ovarioles or germaria, (c) n = 237 (WT), n = 311 (axnSO), n = 239 (axnE77) and n = 119 (apc1apc2) FSCs, (d) n = 75 (WT), n = 67 (axnSO), n = 64 (axnE77) and n = 63 (apc1apc2) biologically independent germaria, (e,f) n = 78 (WT) and n = 89 (arr) biologically independent ovarioles or germaria, (g) n = 228 (WT) and n = 189 (arr) FSCs, (h) n = 112 (WT), n = 132 (axnSO), n = 104 (axnE77) and n = 68 (apc1apc2) layer 1 FSCs, n = 197 (WT), n = 321 (axnSO), n = 237 (axnE77) and n = 125 (apc1apc2) total FSCs, (i) n = 136 (WT) and n = 134 (arr) layer 1 FSCs, n = 228 (WT) and n = 189 (arr) total FSCs. (b,f) Significant differences from controls (WT) were assessed by Pearson’s chi-squared test (∗P < 0.005, #P < 0.05). (a,c,d,e,g,h,i) Significant differences from controls (WT) were assessed by Fisher’s exact two-tailed test (* p < 0.001, # p < 0.05). See Supplementary Table 1 for supporting data.
Supplementary Figure 9 Original images for panels with more than one z-section or image edges.
(a–c,h,i,j–n,u–x) Original images for Figure panels where a diagonal white line indicates the edge of an original image. (d–i,o–t,w,x) Original images for each z-section for Figure panels where different z-sections are shown divided by vertical white lines. Some panels were derived from different z-sections showing neighbouring segments of an ovariole because different z-sections were optimal for highlighting the key colours present in a given egg chamber. In all cases, original images were rotated, if necessary, to give a conventional orientation in final panels of anterior to the left.
Supplementary information
Supplementary Information
Supplementary Information (PDF 24376 kb)
Supplementary Table 1
Supplementary Information (XLSX 29 kb)
(corresponding to Fig. 3a). Radial movement of FSCs.
Maximum projections of the top 3 to 6 z-sections to show relationships of 3 presumed FSCs indicated by arrows. We included 3 to 6 z-sections in a projection in order to capture the highlighted cells throughout the imaging period despite their movement between z-sections. The projections consequently capture only one dimension of the cells’ radial movement. All cells moved regardless of radial position, but since cells in the middle z stacks principally move up and down through z stacks their movement is not captured in this compressed 2-dimensional video. The ‘white’ cell (marked by the white arrow) moved further around the germarium at 4 h 15 min and was temporarily lost from the movie. At 4 h 15 min, the ‘yellow’ cell divided; both daughters are subsequently indicated by yellow arrows. Additional presumed FSCs moved into view from the other side of the gemarium beginning at 3 h 45 min but are not indicated by arrows. At 6 h the germarium began to disintegrate as cells moved out of the posterior half. We have observed twisting rotations in germaria as they appear to attempt egg chamber budding and during these rotations cells move out of the germaria. Bar, 20 μm. (AVI 22197 kb)
(corresponding to Fig. 3b). Radial movement of FSCs.
Maximum projections of the top 2 or 3 z-sections to show the three cells indicated by arrows throughout the movie. The ‘white’ and ‘yellow’ cells crossed one another radially. After 3 h of imaging, cells began to move out of the germarium. Bar, 20 μm. (AVI 9987 kb)
(corresponding to Fig. 4i). FSC movement into EC territory.
Maximum projections of the top 2–4 z stacks are shown. The ‘white’ and ‘magenta’ cells moved anterior into Escort Cell territory. The ‘white’ and ‘red’ cells crossed paths radially at 2 h and again at 4 h. (AVI 10913 kb)
(corresponding to Supplementary Fig. 4a). FSC movement into EC territory.
Two labeled cells started in the 2a/b region and the ‘white’ cell moved anterior into Escort Cell territory. (AVI 8732 kb)
(corresponding to Supplementary Fig. 4b). FSC movement into EC territory.
Maximum projections of the top 4–6 z-sections are shown in order to include the 5 cells indicated by coloured arrows. All cells moved back and forth in radial movements and the ‘white’ cell moved anteriorly into Escort Cell territory. The ‘red’ and ‘yellow’ cells crossed radially. (AVI 12352 kb)
(corresponding to Supplementary Fig. 4c). FSC movement into EC territory.
Maximum projections of all z-sections are shown. Initially the ‘red’, ‘yellow’, and ‘magenta’ cells were in the top half (5 z-sections), whereas the ‘white’ cell was in the last z-section (on the other side of the germarium). The ‘magenta’ cell travelled around to the other side of the germarium. Both white and magenta cells moved anterior into Escort Cell territory. (AVI 7176 kb)
Rights and permissions
About this article
Cite this article
Reilein, A., Melamed, D., Park, K. et al. Alternative direct stem cell derivatives defined by stem cell location and graded Wnt signalling. Nat Cell Biol 19, 433–444 (2017). https://doi.org/10.1038/ncb3505
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb3505
This article is cited by
-
Single-cell expression profile of Drosophila ovarian follicle stem cells illuminates spatial differentiation in the germarium
BMC Biology (2023)
-
MiMIC analysis reveals an isoform specific role for Drosophila Musashi in follicle stem cell maintenance and escort cell function
Cell Death Discovery (2022)
-
Investigating Adult Stem Cells Through Lineage analyses
Stem Cell Reviews and Reports (2022)
-
Special vulnerability of somatic niche cells to transposable element activation in Drosophila larval ovaries
Scientific Reports (2020)
-
A single-cell atlas and lineage analysis of the adult Drosophila ovary
Nature Communications (2020)