Abstract
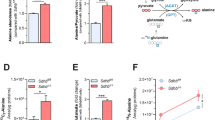
Succinate dehydrogenase (SDH) is a heterotetrameric nuclear-encoded complex responsible for the oxidation of succinate to fumarate in the tricarboxylic acid cycle. Loss-of-function mutations in any of the SDH genes are associated with cancer formation. However, the impact of SDH loss on cell metabolism and the mechanisms enabling growth of SDH-defective cells are largely unknown. Here, we generated Sdhb-ablated kidney mouse cells and used comparative metabolomics and stable-isotope-labelling approaches to identify nutritional requirements and metabolic adaptations to SDH loss. We found that lack of SDH activity commits cells to consume extracellular pyruvate, which sustains Warburg-like bioenergetic features. We further demonstrated that pyruvate carboxylation diverts glucose-derived carbons into aspartate biosynthesis, thus sustaining cell growth. By identifying pyruvate carboxylase as essential for the proliferation and tumorigenic capacity of SDH-deficient cells, this study revealed a metabolic vulnerability for potential future treatment of SDH-associated malignancies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Gaude, E. & Frezza, C. Defects in mitochondrial metabolism and cancer. Cancer Metab. 2, 10 (2014).
Baysal, B. E. et al. Mutations in SDHD, a mitochondrial complex II gene, in hereditary paraganglioma. Science 287, 848–851 (2000).
Niemann, S. & Müller, U. Mutations in SDHC cause autosomal dominant paraganglioma, type 3. Nat. Genet. 26, 268–270 (2000).
Astuti, D. et al. Gene mutations in the succinate dehydrogenase subunit SDHB cause susceptibility to familial pheochromocytoma and to familial paraganglioma. Am. J. Hum. Genet. 69, 49–54 (2001).
Baysal, B. E. et al. Prevalence of SDHB, SDHC, and SDHD germline mutations in clinic patients with head and neck paragangliomas. J. Med. Genet. 39, 178–183 (2002).
Ni, Y. et al. Germline mutations and variants in the succinate dehydrogenase genes in Cowden and Cowden-like syndromes. Am. J. Hum. Genet. 83, 261–268 (2008).
Burnichon, N. et al. SDHA is a tumour suppressor gene causing paraganglioma. Hum. Mol. Genet. 19, 3011–3020 (2010).
Frezza, C., Pollard, P. J. & Gottlieb, E. Inborn and acquired metabolic defects in cancer. J. Mol. Med. 89, 213–220 (2011).
Gill, A. J. et al. Renal tumours and hereditary pheochromocytoma-paraganglioma syndrome type 4. N. Engl. J. Med. 364, 885–886 (2011).
Ricketts, C. J. et al. Succinate dehydrogenase kidney cancer: an aggressive example of the Warburg effect in cancer. J. Urol. 188, 2063–2071 (2012).
Williamson, S. R. et al. Succinate dehydrogenase-deficient renal cell carcinoma: detailed characterization of 11 tumours defining a unique subtype of renal cell carcinoma. Mod. Pathol. 28, 80–94 (2015).
Vanharanta, S. et al. Early-onset renal cell carcinoma as a novel extraparaganglial component of SDHB-associated heritable paraganglioma. Am. J. Hum. Genet. 74, 153–159 (2004).
Ricketts, C. et al. Germline SDHB mutations and familial renal cell carcinoma. J. Natl Cancer Inst. 100, 1260–1262 (2008).
Selak, M. A. et al. Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-α prolyl hydroxylase. Cancer Cell 7, 77–85 (2005).
Xiao, M. et al. Inhibition of α-KG-dependent histone and DNA demethylases by fumarate and succinate that are accumulated in mutations of FH and SDH tumour suppressors. Genes Dev. 26, 1326–1338 (2012).
Letouzé, E. et al. SDH mutations establish a hypermethylator phenotype in paraganglioma. Cancer Cell 10, 739–752 (2013).
Guzy, R. D., Sharma, B., Bell, E., Chandel, N. S. & Schumacker, P. T. Loss of the SdhB, but not the SdhA, subunit of complex II triggers reactive oxygen species-dependent hypoxia-inducible factor activation and tumourigenesis. Mol. Cell. Biol. 28, 718–731 (2008).
Frezza, C. et al. Haem oxygenase is synthetically lethal with the tumour suppressor fumarate hydratase. Nature 17, 225–228 (2011).
Zheng, L. et al. Reversed argininosuccinate lyase activity in fumarate hydratase-deficient cancer cells. Cancer Metab. 1, 1–12 (2013).
Zheng, L. et al. Fumarate induces redox-dependent senescence by modifying glutathione metabolism. Nat. Commun. 6, 6001 (2015).
Housley, S. L. et al. Renal carcinoma with giant mitochondria associated with germ-line mutation and somatic loss of the succinate dehydrogenase B gene. Histopathology 56, 405–408 (2010).
Favier, J. et al. Warburg effect is genetically determined in inherited pheochromocytomas. PLoS ONE 4, e7094 (2009).
Mullen, A. R. et al. Oxidation of α-ketoglutarate is required for reductive carboxylation in cancer cells with mitochondrial defects. Cell Rep. 7, 1679–1690 (2014).
Yang, C. et al. Metabolic reprogramming for producing energy and reducing power in fumarate hydratase null cells from hereditary leiomyomatosis renal cell carcinoma. PLoS ONE 8, e72179 (2013).
Folger, O. et al. Predicting selective drug targets in cancer through metabolic networks. Mol. Syst. Biol. 7, 501 (2011).
Cheng, T. et al. Pyruvate carboxylase is required for glutamine-independent growth of tumour cells. Proc. Natl Acad. Sci. USA 108, 8674–8679 (2011).
Jitrapakdee, S. & Wallace, J. C. Structure, function and regulation of pyruvate carboxylase. Biochem. J. 340, 1–16 (1999).
Sellers, K. et al. Pyruvate carboxylase is critical for non-small-cell lung cancer proliferation. J. Clin. Invest. 125, 687–698 (2015).
Izquierdo-Garcia, J. L. et al. Glioma cells with the IDH1 mutation modulate metabolic fractional flux through pyruvate carboxylase. PLoS ONE 9, e108289 (2014).
Liu, P., Jenkins, N. A. & Copeland, N. G. A highly efficient recombineering-based method for generating conditional knockout mutations. Genome Res. 13, 476–484 (2003).
Van der Weyden, L. et al. Null and conditional semaphorin 3B alleles using a flexible puroDeltatk loxP/FRT vector. Genesis 41, 171–178 (2005).
Vintersten, K. et al. Mouse in red: red fluorescent protein expression in mouse ES cells, embryos, and adult animals. Genesis 40, 241–246 (2004).
Tucker, K. L., Wang, Y., Dausman, J. & Jaenisch, R. A transgenic mouse strain expressing four drug-selectable marker genes. Nucleic Acids Res. 25, 3745–3746 (1997).
Nagy, A., Gertsenstein, M., Vintersten, K. & Behringer, R. Manipulating the Mouse Embryo: A Laboratory Manual 3rd edn 453–506 (Cold Spring Harbor Press, 2003).
Mathew, R., Degenhardt, K., Haramaty, L., Karp, C. M. & White, E. Immortalized mouse epithelial cell models to study the role of apoptosis in cancer. Methods Enzymol. 446, 77–106 (2008).
Zhang, J. et al. Measuring energy metabolism in cultured cells, including human pluripotent stem cells and differentiated cells. Nat. Protoc. 7, 1068–1085 (2012).
Spinazzi, M., Casarin, A., Pertegato, V., Salviati, L. & Angelini, C. Assessment of mitochondrial respiratory chain enzymatic activities on tissues and cultured cells. Nat. Protoc. 7, 12235–12246 (2012).
Mitra, K. & Lippincott-Schwartz, J. Analysis of mitochondrial dynamics and functions using imaging approaches. Curr. Protoc. Cell. Biol. 4, 1–21 (2010).
Gonzalvez, F. et al. Cardiolipin provides an essential activating platform for caspase-8 on mitochondria. J. Cell Biol. 183, 681–696 (2008).
Kamphorst, J. J., Chung, M. K., Fan, J. & Rabinowitz, J. D. Quantitative analysis of acetyl-CoA production in hypoxic cancer cells reveals substantial contribution from acetate. Cancer Metab. 2, 23 (2014).
Smart, K. F., Aggio, R. B. M., Houtte, J. R. V. & Villas-Boas, S. G. Analytical platform for metabolome analysis of microbial cells using methyl chloroformate derivatisation followed by gas chromatography-mass spectrometry. Nat. Protoc. 5, 1709–1729 (2010).
Ringnér, M. What is principal component analysis? Nat. Biotechnol. 26, 303–304 (2008).
Acknowledgements
We would like to acknowledge S. Tardito and Z. T. Schug for comments and interpretation of the results, A. King for editorial work, U. Srirangalingam for help in human specimen collection and the Beatson Institute mouse facility staff for housing of mice and xenograft measurements.
Author information
Authors and Affiliations
Contributions
S.C. conceived the study, designed and carried out the experiments, interpreted the data and wrote the manuscript; L.Z. carried out the untargeted metabolomic analysis; G.M., N.J.F.v.d.B. S.T., V.B., J.J.K. and A.V. supervised the targeted LC-MS and GC-MS analyses; E.D.M. supervised the generation of kidney cells; D. Strathdee and D. Stevenson generated genetically modified Sdhbfl/fl mice; G.K. carried out the bioinformatics and statistical analyses; C.N. carried out immunohistochemistry of human SDHB-associated RCC; S.F. carried out histopathological analysis of SDHB-related RCC; F.S. collected and provided human paraganglioma and pheochromocytoma samples; K.B. supervised animal work; E.G. conceived and supervised the study, interpreted the data and revised the manuscript. All the authors discussed the results and commented on the manuscript. This work was funded by Cancer Research UK. S.C. is the recipient of a FEBS long-term fellowship.
Corresponding author
Ethics declarations
Competing interests
E.G. is a shareholder and a consultant of MetaboMed Israel, Ltd.
Integrated supplementary information
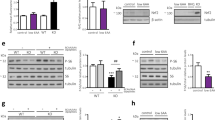
Supplementary Figure 1 Generation of Sdhb-deficient cells.
(a) Schematic representation of the mouse Sdhb genomic locus and Sdhb targeted allele. Numbers specify exons, arrowheads indicate LoxP sites; Hyg, Hygromycin-resistance gene cassette. 3 LoxP U1, 3 LoxP D1 and Hyg U1 primers were used for genotyping. (b) Bright-field images of Sdhbfl/fl and SdhbΔ/Δ cells. Scale bar = 280 μm. (c) PCR analysis of gDNA of kidney epithelial cells of the indicated genotypes after infection (Cre) with Ad5-CMV-Cre-GFP; bp, base pairs. (d) Western blot analysis of SDHB levels of the indicated cells. Data in b-d derive from one experiment, performed once. (e) Determination of SDH activity of the indicated cells in the presence (+) or absence (−) of 20 mM malonate. Data are presented as mean ± s.e.m. of n = 3 wells pooled from three independent experiments. (f) % labelling of lipogenic AcCoA from glucose and glutamine. Data are presented as mean ± s.e.m. (n = 3 wells) of one experiment, performed once. Raw data of independently repeated experiments are provided in Supplementary Table 3.
Supplementary Figure 2 Sdhb loss induces Warburg-like bioenergetic features.
(a) Maximal and ATP-coupled respiration. Dashed line indicates the relative basal mitochondrial OCR. Data are presented as mean ± s.e.m. of three reading cycles of n = 27 wells pooled from four independent experiments. (b) Mitochondrial mass; a.u., arbitrary units. Data are presented as mean ± s.e.m. of n = 3 wells pooled from three independent experiments. (c) Representative Western blot analysis (out of 2 independent experiments) of the levels of two complex I subunits (red) and several mitochondrial proteins (green) assessed in the indicated mitochondrial extracts. (CI, Complex I; CVa, ComplexVa, CIII Core1, Complex III Core 1, CypD, Cyclophilin D, Cyt c, Cytochrome c, COX IV, Cytochrome c Oxidase). (d) Mitochondrial Complex I activity. Data are presented as mean ± s.e.m. (n = 4 wells) of one experiment, performed once. (e,f) AMP/ATP ratio (expressed as fold change compared to untreated Sdhfl/fl cells), AMP and ATP levels (expressed as % of untreated Sdhfl/fl cells) upon treatment of the indicated cells with 0.5 μM oligomycin for 8 h. (g) ECAR of the indicated cells. Data are presented as mean ± s.e.m. of n = 21 (Sdhbfl/fl) and n = 23 (SdhbΔ/Δ) wells pooled from three independent experiments. (h) Lactate secretion by Sdhbfl/fl and SdhbΔ/Δ cells cultured for 48 h with U-13C-glucose in presence/absence of 5 mM oxamate. The sum of all isotopologues is reported for clarity. Data are presented as mean ± s.e.m. (n = 6 wells) of one experiment, performed once. (i,j) AMP/ATP ratio (expressed as fold change compared to untreated Sdhfl/fl cells), AMP and ATP levels (expressed as % of untreated Sdhfl/fl cells) upon treatment with 5 mM oxamate for 24 h. (k) NAD+ and NADH levels (expressed as % of Pyruvate-fed Sdhfl/fl cells) in cells incubated for 24 h in the presence/absence of pyruvate. Data in e, f, k are presented as mean ± s.e.m. (n = 3 wells) of one representative experiment, independently replicated twice. (l,m) NAD+/NADH ratio (expressed as fold change compared to untreated Sdhfl/flcells), NAD+ and NADH levels (expressed as % of untreated Sdhfl/fl cells) in cells incubated for 24 h in the presence/absence of 5 mM oxamate. Data in i, j, l, m are presented as mean ± s.e.m. (n = 3 wells) of one experiment, performed once. Raw data of independently repeated experiments are provided in Supplementary Table 3.
Supplementary Figure 3 Pyruvate-dependency of Sdhb-deficient cells.
(a) Determination of secretion (positive bars) and consumption (negative bars) rates of the indicated metabolites of cells cultured for 48 h with U-13C-glucose. The sum of all isotopologues is reported for clarity. Data are presented as mean ± s.e.m. of n = 18 wells pooled from three independent experiments. (b) Isotopologues labeling profile of intracellular pyruvate in cells incubated for 24 h with U-13C-pyruvate. (c) Labeling of pyruvate after incubation of cells for 24 h with with U-13C-Glucose in presence (+) or absence (−) of pyruvate. Data in b, c are presented as mean ± s.e.m. (n = 3 wells) of one representative experiment, independently replicated twice. Raw data of independently repeated experiments are provided in Supplementary Table 3.
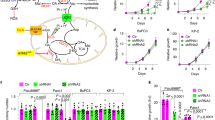
Supplementary Figure 4 Essentiality of pyruvate carboxylase for growth of Sdhb-deficient cells.
(a) Immuhistochemical assessment of PC levels in two human SDHB-related RCC samples. N.A.T., normal adjacent tissue; T., tumour. Scale bars = 100 μM. Numbers on the left indicates the Tumour ID described in Supplementary Table 2. (b) qPCR analysis of Pcx expression in cells infected with lentiviruses expressing either a non-targeting shRNAsequence (shNTC) or an shRNA targeting Pcx. (c) Measurement of malate levels in cells treated as in (b) cultured for 24 h in presence of U-13C-glucose. Data in b, c are presented as mean ± s.e.m.of n = 9 wells pooled from three independent experiments. (d) Abundance of 13C1-malate in cells treated as in (b) incubated for 10 min in presence of13C-bicarbonate. Data are presented as mean ± s.e.m. (n = 3 wells) of one representative experiment, independently replicated twice. (e) Measurement of Aspartate levels in cells treated as in (b) cultured for 24 h in presence of U-13C-glutamine. (f) PCX expression inH-RasV 12-transformed cells infected with lentiviruses expressing either a shNTC or shPcx-5 sequence before injection into nude mice. Data in e, f are presented as mean ± s.e.m. (n = 3 wells) of one representative experiment, performed once. (g–i) In vivo growth of cells described in (f) xenografted in athymic nude mice. The % of tumour-free mice over time (g) and the tumour volumes of each xenografted mouse (n = 10 mice per group) (h,i) are presented. The Log-rank (Mantel–Cox) test was used to calculate the statistical significance between curves in (g). A statistical permutation test was used to compare the statistical significance between curves of the selected genotypes in (h) and (i), as described in Methods. Data derive fromone experiment, performed once. Raw data of independently repeated experiments are provided in Supplementary Table 3.
Supplementary Figure 5 Pyruvate carboxylation sustains aspartate biosynthesis.
(a) Measurement of citrate levels in cells cultured for 24 h in presence of U-13C-glucose. (b) Number of control and PCX-silenced cells supplemented with 2.5 mM aspartate measured after 96 h of culture. Data are presented as mean ± s.e.m. (n = 4 wells) of one representative experiment, performed once. Data related to shNTC control cells are shared with Fig. 6c (c) Determination of total intracellular palmitate levels in PCX-silenced cells supplemented with 50 μM palmitate for 24 h. Data in a, c are presented as mean ± s.e.m. (n = 3 wells) of one representative experiment, performed once. Raw data of independently repeated experiments are provided in Supplementary Table 3.
Supplementary information
Supplementary Information
Supplementary Information (PDF 555 kb)
Supplementary Information
Supplementary Table 1 (XLSX 404 kb)
Supplementary Information
Supplementary Table 2 (XLSX 9 kb)
Supplementary Information
Supplementary Table 3 (XLSX 190 kb)
Rights and permissions
About this article
Cite this article
Cardaci, S., Zheng, L., MacKay, G. et al. Pyruvate carboxylation enables growth of SDH-deficient cells by supporting aspartate biosynthesis. Nat Cell Biol 17, 1317–1326 (2015). https://doi.org/10.1038/ncb3233
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb3233
This article is cited by
-
Pyruvate anaplerosis is a targetable vulnerability in persistent leukaemic stem cells
Nature Communications (2023)
-
Multifaceted mitochondria: moving mitochondrial science beyond function and dysfunction
Nature Metabolism (2023)
-
Pan-tissue mitochondrial phenotyping reveals lower OXPHOS expression and function across cancer types
Scientific Reports (2023)
-
Viability of HepG2 and MCF-7 cells is not correlated with mitochondrial bioenergetics
Scientific Reports (2023)
-
Fumarate induces vesicular release of mtDNA to drive innate immunity
Nature (2023)