Abstract
Robust mechanisms to control cell proliferation have evolved to maintain the integrity of organ architecture. Here, we investigated how two critical proliferative pathways, Myc and E2f, are integrated to control cell cycles in normal and Rb-deficient cells using a murine intestinal model. We show that Myc and E2f1–3 have little impact on normal G1–S transitions. Instead, they synergistically control an S–G2 transcriptional program required for normal cell divisions and maintaining crypt–villus integrity. Surprisingly, Rb deficiency results in the Myc-dependent accumulation of E2f3 protein and chromatin repositioning of both Myc and E2f3, leading to the ‘super activation’ of a G1–S transcriptional program, ectopic S phase entry and rampant cell proliferation. These findings reveal that Rb-deficient cells hijack and redeploy Myc and E2f3 from an S–G2 program essential for normal cell cycles to a G1–S program that re-engages ectopic cell cycles, exposing an unanticipated addiction of Rb-null cells on Myc.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Bertoli, C., Skotheim, J. M. & de Bruin, R. A. Control of cell cycle transcription during G1 and S phases. Nat. Rev. Mol. Cell Biol. 14, 518–528 (2013).
Meyer, N. & Penn, L. Z. Reflecting on 25 years with MYC. Nat. Rev. Cancer 8, 976–990 (2008).
Palomero, T. et al. NOTCH1 directly regulates c-MYC and activates a feed-forward-loop transcriptional network promoting leukemic cell growth. Proc. Natl Acad. Sci. USA 103, 18261–18266 (2006).
Sharma, V. M. et al. Notch1 contributes to mouse T-cell leukemia by directly inducing the expression of c-myc. Mol. Cell Biol. 26, 8022–8031 (2006).
Weng, A. P. et al. c-Myc is an important direct target of Notch1 in T-cell acute lymphoblastic leukemia/lymphoma. Genes Dev. 20, 2096–2109 (2006).
He, T. C. et al. Identification of c-MYC as a target of the APC pathway. Science 281, 1509–1512 (1998).
Seoane, J. et al. TGFβ influences Myc, Miz-1 and Smad to control the CDK inhibitor p15INK4b. Nat. Cell Biol. 3, 400–408 (2001).
Staller, P. et al. Repression of p15INK4b expression by Myc through association with Miz-1. Nat. Cell Biol. 3, 392–399 (2001).
Leone, G., DeGregori, J., Sears, R., Jakoi, L. & Nevins, J. R. Myc and Ras collaborate in inducing accumulation of active cyclin E/Cdk2 and E2F. Nature 387, 422–426 (1997).
Bouchard, C. et al. Direct induction of cyclin D2 by Myc contributes to cell cycle progression and sequestration of p27. EMBO J. 18, 5321–5333 (1999).
Hermeking, H. et al. Identification of CDK4 as a target of c-MYC. Proc. Natl Acad. Sci. USA 97, 2229–2234 (2000).
Gomez-Roman, N., Grandori, C., Eisenman, R. N. & White, R. J. Direct activation of RNA polymerase III transcription by c-Myc. Nature 421, 290–294 (2003).
Arabi, A. et al. c-Myc associates with ribosomal DNA and activates RNA polymerase I transcription. Nat. Cell Biol. 7, 303–310 (2005).
Grandori, C. et al. c-Myc binds to human ribosomal DNA and stimulates transcription of rRNA genes by RNA polymerase I. Nat. Cell Biol. 7, 311–318 (2005).
Grewal, S. S., Li, L., Orian, A., Eisenman, R. N. & Edgar, B. A. Myc-dependent regulation of ribosomal RNA synthesis during Drosophila development. Nat. Cell Biol. 7, 295–302 (2005).
Zhang, H. et al. HIF-1 inhibits mitochondrial biogenesis and cellular respiration in VHL-deficient renal cell carcinoma by repression of C-MYC activity. Cancer Cell 11, 407–420 (2007).
Morrish, F., Neretti, N., Sedivy, J. M. & Hockenbery, D. M. The oncogene c-Myc coordinates regulation of metabolic networks to enable rapid cell cycle entry. Cell Cycle 7, 1054–1066 (2008).
Dominguez-Sola, D. et al. Non-transcriptional control of DNA replication by c-Myc. Nature 448, 445–451 (2007).
Sears, R., Ohtani, K. & Nevins, J. R. Identification of positively and negatively acting elements regulating expression of the E2F2 gene in response to cell growth signals. Mol. Cell Biol. 17, 5227–5235 (1997).
Adams, M. R., Sears, R., Nuckolls, F., Leone, G. & Nevins, J. R. Complex transcriptional regulatory mechanisms control expression of the E2F3 locus. Mol. Cell Biol. 20, 3633–3639 (2000).
Trimarchi, J. M. & Lees, J. A. Sibling rivalry in the E2F family. Nat. Rev. Mol. Cell Biol. 3, 11–20 (2002).
Dimova, D. K. & Dyson, N. J. The E2F transcriptional network: old acquaintances with new faces. Oncogene 24, 2810–2826 (2005).
Chen, H. Z., Tsai, S. Y. & Leone, G. Emerging roles of E2Fs in cancer: an exit from cell cycle control. Nat. Rev. Cancer 9, 785–797 (2009).
Dang, C. V. MYC on the path to cancer. Cell 149, 22–35 (2012).
Bettess, M. D. et al. c-Myc is required for the formation of intestinal crypts but dispensable for homeostasis of the adult intestinal epithelium. Mol. Cell Biol. 25, 7868–7878 (2005).
Li, F. et al. Conditional deletion of c-myc does not impair liver regeneration. Cancer Res. 66, 5608–5612 (2006).
Chen, D. et al. Division and apoptosis of E2f-deficient retinal progenitors. Nature 462, 925–929 (2009).
Chong, J. L. et al. E2f1-3 switch from activators in progenitor cells to repressors in differentiating cells. Nature 462, 930–934 (2009).
Wenzel, P. L. et al. Cell proliferation in the absence of E2F1-3. Dev. Biol. 351, 35–45 (2011).
Malynn, B. A. et al. N-myc can functionally replace c-myc in murine development, cellular growth, and differentiation. Genes Dev. 14, 1390–1399 (2000).
Tsai, S. Y. et al. Mouse development with a single E2F activator. Nature 454, 1137–1141 (2008).
Ireland, H. et al. Inducible Cre-mediated control of gene expression in the murine gastrointestinal tract: effect of loss of β-catenin. Gastroenterology 126, 1236–1246 (2004).
Muncan, V. et al. Rapid loss of intestinal crypts upon conditional deletion of the Wnt/Tcf-4 target gene c-Myc. Mol. Cell Biol. 26, 8418–8426 (2006).
Sansom, O. J. et al. Myc deletion rescues Apc deficiency in the small intestine. Nature 446, 676–679 (2007).
McGarry, T. J. & Kirschner, M. W. Geminin, an inhibitor of DNA replication, is degraded during mitosis. Cell 93, 1043–1053 (1998).
Ireland, H., Houghton, C., Howard, L. & Winton, D. J. Cellular inheritance of a Cre-activated reporter gene to determine Paneth cell longevity in the murine small intestine. Dev. Dyn. 233, 1332–1336 (2005).
Phesse, T. J. et al. Endogenous c-Myc is essential for p53-induced apoptosis in response to DNA damage in vivo. Cell Death Differ. 21, 956–966 (2014).
Rhee, H. S. & Pugh, B. F. Comprehensive genome-wide protein-DNA interactions detected at single-nucleotide resolution. Cell 147, 1408–1419 (2011).
Serandour, A. A., Brown, G. D., Cohen, J. D. & Carroll, J. S. Development of an Illumina-based ChIP-exonuclease method provides insight into FoxA1-DNA binding properties. Genome Biol. 14, R147 (2013).
Gauthier, N. P. et al. Cyclebase.org–a comprehensive multi-organism online database of cell-cycle experiments. Nucleic Acids Res. 36, D854-9 (2008).
Landis, M. W., Pawlyk, B. S., Li, T., Sicinski, P. & Hinds, P. W. Cyclin D1-dependent kinase activity in murine development and mammary tumorigenesis. Cancer Cell 9, 13–22 (2006).
Yu, Q. et al. Requirement for CDK4 kinase function in breast cancer. Cancer Cell 9, 23–32 (2006).
Malumbres, M. & Barbacid, M. Cell cycle, CDKs and cancer: a changing paradigm. Nat. Rev. Cancer 9, 153–166 (2009).
Rustgi, A. K., Dyson, N. & Bernards, R. Amino-terminal domains of c-myc and N-myc proteins mediate binding to the retinoblastoma gene product. Nature 352, 541–544 (1991).
Goodrich, D. W. & Lee, W. H. Abrogation by c-myc of G1 phase arrest induced by RB protein but not by p53. Nature 360, 177–179 (1992).
Heinen, C. D. et al. The APC tumor suppressor controls entry into S-phase through its ability to regulate the cyclin D/RB pathway. Gastroenterology 123, 751–763 (2002).
Morris, E. J. et al. E2F1 represses β-catenin transcription and is antagonized by both pRB and CDK8. Nature 455, 552–556 (2008).
Davidson, G. et al. Cell cycle control of wnt receptor activation. Dev. Cell 17, 788–799 (2009).
Cole, A. M. et al. Cyclin D2-cyclin-dependent kinase 4/6 is required for efficient proliferation and tumorigenesis following Apc loss. Cancer Res. 70, 8149–8158 (2010).
Leone, G. et al. Identification of a novel E2F3 product suggests a mechanism for determining specificity of repression by Rb proteins. Mol. Cell Biol. 20, 3626–3632 (2000).
McMahon, S. B., Van Buskirk, H. A., Dugan, K. A., Copeland, T. D. & Cole, M. D. The novel ATM-related protein TRRAP is an essential cofactor for the c-Myc and E2F oncoproteins. Cell 94, 363–374 (1998).
Ogawa, H., Ishiguro, K., Gaubatz, S., Livingston, D. M. & Nakatani, Y. A complex with chromatin modifiers that occupies E2F- and Myc-responsive genes in G0 cells. Science 296, 1132–1136 (2002).
Yang, Z. F., Mott, S. & Rosmarin, A. G. The Ets transcription factor GABP is required for cell-cycle progression. Nat. Cell Biol. 9, 339–346 (2007).
Uribesalgo, I. et al. E-box-independent regulation of transcription and differentiation by MYC. Nat. Cell Biol. 13, 1443–1449 (2011).
Lin, C. Y. et al. Transcriptional amplification in tumor cells with elevated c-Myc. Cell 151, 56–67 (2012).
Nie, Z. et al. c-Myc is a universal amplifier of expressed genes in lymphocytes and embryonic stem cells. Cell 151, 68–79 (2012).
Sabo, A. et al. Selective transcriptional regulation by Myc in cellular growth control and lymphomagenesis. Nature 511, 488–492 (2014).
Walz, S. et al. Activation and repression by oncogenic MYC shape tumour-specific gene expression profiles. Nature 511, 483–487 (2014).
Nielsen, S. J. et al. Rb targets histone H3 methylation and HP1 to promoters. Nature 412, 561–565 (2001).
Narita, M. et al. Rb-mediated heterochromatin formation and silencing of E2F target genes during cellular senescence. Cell 113, 703–716 (2003).
Siddiqui, H., Fox, S. R., Gunawardena, R. W. & Knudsen, E. S. Loss of RB compromises specific heterochromatin modifications and modulates HP1α dynamics. J. Cell Physiol. 211, 131–137 (2007).
Petrella, L. N. et al. synMuv B proteins antagonize germline fate in the intestine and ensure C. elegans survival. Development 138, 1069–1079 (2011).
Trumpp, A. et al. c-Myc regulates mammalian body size by controlling cell number but not cell size. Nature 414, 768–773 (2001).
Saenz-Robles, M. T. et al. Intestinal hyperplasia induced by simian virus 40 large tumor antigen requires E2F2. J. Virol. 81, 13191–13199 (2007).
Langmead, B. & Salzberg, S. L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 9, 357–359 (2012).
Guo, Y., Mahony, S. & Gifford, D. K. High resolution genome wide binding event finding and motif discovery reveals transcription factor spatial binding constraints. PLoS Comput. Biol. 8, e1002638 (2012).
Zhang, Y. et al. Model-based analysis of ChIP-Seq (MACS). Genome Biol. 9, R137 (2008).
Heinz, S. et al. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol. Cell 38, 576–589 (2010).
Robinson, J. T. et al. Integrative genomics viewer. Nat. Biotechnol. 29, 24–26 (2011).
Acknowledgements
We thank M. L. Rawahneh, N. Lovett and J. Moffitt for assistance with histology. We also acknowledge assistance from C. Martin for reviewing histological slides, S. Bae for advice on sequencing library construction, and V. Jin, Z. Ye and J. McElroy for suggestions on the analysis of sequencing data. This work was supported by the Microarray, Nucleic Acid and Analytical Cytometry Core Shared Resources at Ohio State University. We are grateful to V. Gopalan and A. Simcox for critical suggestions. This work was financially supported by NIH grants to G.L. (R01CA121275 and R01HD047470) and an NIH grant to J.M.P. (R01CA098956). H.L., T.P. and B.H. were recipients of Graduate, Postdoctoral and Undergraduate Pelotonia Fellowships, respectively.
Author information
Authors and Affiliations
Contributions
H.L. and G.L. designed the experiments. H.L., P.D., B.H., S.R., N.F., A.B., R.K., C.K. and M.T.S.-R. performed the experiments, collected and analysed data. X.T., A.S., T.P., K.H., R.M. and P.C. performed bioinformatic and statistical analysis for the gene expression and ChIP-exo-seq data. Z.C. and Q.W. advised for the ChIP-exo-seq experiments. J.M.P. and G.L. supervised the study. H.L. and G.L. wrote the manuscript with inputs from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Loss of E2f1-3 and/or Myc in crypts.
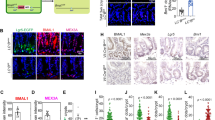
(a) Dual staining of EdU (red) and geminin (green, included here for the composition of merged images) in crypts from intestines harvested 2 days after induction of Ah-cre expression. Nuclei were stained with DAPI (blue). Data are representative images from n = 3 mice per genetic group. (b) Quantification of EdU and geminin staining. Data presented as mean ± s.d., n = 3 mice per genetic group. (c) IHC staining of Myc in intestines harvested 2 days after induction of Ah-cre expression. Data are representative images from n = 3 mice for each genetic group. (d) Alcian Blue staining for goblet cells (blue) and IF staining of lysozyme for paneth cells (red) in intestines harvested 2 days after induction of Ah-cre expression. Nuclei were counterstained with Nuclear Fast Red or DAPI. Data are representative images from n = 3 mice per genetic group. Scale bars in a, c and d represent 50 μm.
Supplementary Figure 2 DNA binding of E2f3 and Myc in wild type crypts.
(a) IHC staining of E2f3 using the antibody (Millipore; 05-551) in control, E2f3a−/−, E2f3b−/− and Ah-cre;E2f1−/−;E2f2−/−;E2f3loxP/loxP intestines. Data are representative images from n = 2 mice per genetic group. E2f3b−/− but not E2f3a−/− intestines show positive-stained cells, indicating the specific recognition of E2f3a isoform by this antibody. Note the non-specific staining of blood cells. (b) Heatmap representation of differentially expressed genes in wild type crypts (n = 5) versus wild type villi (n = 5). P < 0.01, empirical Bayes method. (c) ChIP-exo-seq track examples showing E2f3 and Myc binding to selected G1-S and S-G2 related genes in wild type crypts. Examples are derived from pooled crypts (n = 32 mice). Scale bars in a represent 25 μm. Scale bars in c represent 1kb.
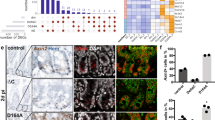
Supplementary Figure 3 Loss of either E2f1-3 or Myc corrects aberrant transcription in Rb KO villi.
RT-qPCR analysis of mRNA levels for a subset of genes in control, Rb KO, Rb/E2f QKO and Rb/Myc DKO villi. Expression levels from individual mice are plotted (2 or 3 per genetic group as indicated) and error bars represent mean ± s.d. from n = 3 technical replicates. The aberrant expression of these genes in Rb KO villi were normalized by loss of either E2f1-3 or Myc. The average expression level of control samples was set as 1.
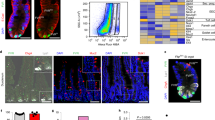
Supplementary Figure 4 Peak summit-distance plots and tag intensity heatmaps across tissue compartments and genetic groups.
(a) Peak summit-distance plots for E2f3 summits that are associated with the 701 dysregulated genes in Rb KO villi. E2f3 binding is compared between crypts and villi of the same genetic group. (b) Heatmap representation of tag intensity for all E2f3 binding locations. E2f3 binding is compared between crypts and villi of the same genetic group. (c) Peak summit-distance plots for E2f3 summits that are associated with the 701 dysregulated genes in Rb KO villi. E2f3 binding is compared between control and Rb KO intestines. (d) Heatmap representation of tag intensity for all E2f3 binding locations. E2f3 binding is compared between control and Rb KO intestines. (e) Peak summit-distance plots for Myc summits that are associated with the 701 dysregulated genes in Rb KO villi. Myc binding is compared between crypts and villi of the same genetic group. (f) Heatmap representation of tag intensity for all Myc binding locations. Myc binding is compared between crypts and villi of the same genetic group. (g) Peak summit-distance plots for Myc summits that are associated with the 701 dysregulated genes in Rb KO villi. Myc binding is compared between control and Rb KO intestines. (h) Heatmap representation of tag intensity for all Myc binding locations. Myc binding is compared between control and Rb KO intestines. Data in this figure (a–h) are derived from pooled crypts (n = 32 mice for control, n = 27 mice for Rb KO) or villi (n = 7 mice for control, n = 7 mice for Rb KO).
Supplementary Figure 5 E2f3 DNA binding in wild type and Rb KO villi.
(a–c) Three main categories of target genes having different patterns of E2f3 DNA binding in wild type and Rb KO villi are illustrated: Group 1 includes target genes with E2f3 peaks present in Rb KO villi but absent in control villi; Group 2 includes target genes with distinct E2f3 peaks in control and Rb KO villi (either due to additional peak summits or peak position changes); Group 3 includes target genes with E2f3 peaks similarly positioned in control and Rb KO villi (but possibly with different magnitude of binding strengths). ChIP-exo-seq track examples are shown for group 1 (a), group 2 (b) and group 3 (c). Data in this figure are derived from pooled villi (n = 7 mice for control, n = 7 mice for Rb KO). Scale bars in a–c represent 1kb.
Supplementary Figure 6 DNA binding strength and DNA binding motifs of all Myc and E2f3 peaks.
(a) Tag intensity plots (tags per bp per peak per 100M reads) around all peak summits identified in crypts and villi, as indicated. (b) Canonical DNA binding motifs (TTCCCGCC motif for E2f3, underlined with blue lines; CACGTG motif for Myc, underlined with reds lines) or strongest non-canonical motifs identified from all peak sequences in indicated genomic regions. Data in this figure are derived from pooled crypts (n = 32 mice for control, n = 27 mice for Rb KO) or villi (n = 7 mice for control, n = 7 mice for Rb KO).
Supplementary Figure 7 DNA binding motifs of Myc and E2f3 peaks associated with the 701 dysregulated genes in Rb KO villi.
Canonical DNA binding motifs (TTCCCGCC motif for E2f3, underlined with blue lines; CACGTG motif for Myc, underlined with reds lines) or strongest non-canonical motifs identified from peak sequences in indicated genomic regions of the 701 dysregulated gene in Rb KO villi. Data in this figure derived from pooled crypts (n = 32 mice for control, n = 27 mice for Rb KO) or villi of (n = 7 mice for control, n = 7 mice for Rb KO).
Supplementary Figure 8 Expression of Wnt/β-catenin targets in Rb KO villi and auto-regulation of E2fs.
(a) Venn diagram showing the overlap between the 701 genes dysregulated in Rb KO villi and the 111 Wnt/β-catenin target genes. (b) ChIP-exo-seq tracks showing E2f3 binding to the E2f1 and E2f2 loci. The differential binding between control and Rb KO villi is highlighted in blue. Examples are derived from pooled villi (n = 7 mice for control, n = 7 mice for Rb KO). Scale bars in b represent 1kb.
Supplementary information
Supplementary Information
Supplementary Information (PDF 2312 kb)
Supplementary Table 1
Supplementary Information (XLSX 411 kb)
Supplementary Table 2
Supplementary Information (XLSX 51 kb)
Supplementary Table 3
Supplementary Information (XLSX 11 kb)
Supplementary Table 4
Supplementary Information (XLSX 859 kb)
Supplementary Table 5
Supplementary Information (XLSX 161 kb)
Supplementary Table 6
Supplementary Information (XLSX 31 kb)
Supplementary Table 7
Supplementary Information (XLSX 297 kb)
Supplementary Table 8
Supplementary Information (XLSX 34 kb)
Supplementary Table 9
Supplementary Information (XLSX 94 kb)
Supplementary Table 10
Supplementary Information (XLSX 9 kb)
Supplementary Table 11
Supplementary Information (XLSX 16 kb)
Rights and permissions
About this article
Cite this article
Liu, H., Tang, X., Srivastava, A. et al. Redeployment of Myc and E2f1–3 drives Rb-deficient cell cycles. Nat Cell Biol 17, 1036–1048 (2015). https://doi.org/10.1038/ncb3210
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb3210
This article is cited by
-
Metformin escape in prostate cancer by activating the PTGR1 transcriptional program through a novel super-enhancer
Signal Transduction and Targeted Therapy (2023)
-
Prognostic analysis of E2F transcription factors E2F1 and E2F3 in four independent pediatric neuroblastoma cohorts
BMC Pediatrics (2022)
-
MYCN induces cell-specific tumorigenic growth in RB1-proficient human retinal organoid and chicken retina models of retinoblastoma
Oncogenesis (2022)
-
E2F4 transcription factor is a prognostic biomarker related to immune infiltration of head and neck squamous cell carcinoma
Scientific Reports (2022)
-
NOTCH target gene HES5 mediates oncogenic and tumor suppressive functions in hepatocarcinogenesis
Oncogene (2020)