Abstract
Coordination of stem cell activity with inflammatory responses is critical for regeneration and homeostasis of barrier epithelia. The temporal sequence of cell interactions during injury-induced regeneration is only beginning to be understood. Here we show that intestinal stem cells (ISCs) are regulated by macrophage-like haemocytes during the early phase of regenerative responses of the Drosophila intestinal epithelium. On tissue damage, haemocytes are recruited to the intestine and secrete the BMP homologue DPP, inducing ISC proliferation by activating the type I receptor Saxophone and the Smad homologue SMOX. Activated ISCs then switch their response to DPP by inducing expression of Thickveins, a second type I receptor that has previously been shown to re-establish ISC quiescence by activating MAD. The interaction between haemocytes and ISCs promotes infection resistance, but also contributes to the development of intestinal dysplasia in ageing flies. We propose that similar interactions influence pathologies such as inflammatory bowel disease and colorectal cancer in humans.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Ferrandon, D. The complementary facets of epithelial host defenses in the genetic model organism Drosophila melanogaster: from resistance to resilience. Curr. Opin. Immunol. 25, 59–70 (2013).
Lemaitre, B. & Miguel-Aliaga, I. The digestive tract of Drosophila melanogaster. Annu. Rev. Genet. 47, 377–404 (2013).
Ostaff, M. J., Stange, E. F. & Wehkamp, J. Antimicrobial peptides and gut microbiota in homeostasis and pathology. EMBO Mol. Med. 5, 1465–1483 (2013).
Kaiko, G. E. & Stappenbeck, T. S. Host–microbe interactions shaping the gastrointestinal environment. Trends Immunol. 35, 538–548 (2014).
Buchon, N., Broderick, N. A. & Lemaitre, B. Gut homeostasis in a microbial world: insights from Drosophila melanogaster. Nat. Rev. Microbiol. 11, 615–626 (2013).
Ayyaz, A. & Jasper, H. Intestinal inflammation and stem cell homeostasis in aging. Front. Cell. Infect. Microbiol. 3, 98 (2013).
Micchelli, C. A. & Perrimon, N. Evidence that stem cells reside in the adult Drosophila midgut epithelium. Nature 439, 475–479 (2006).
Ohlstein, B. & Spradling, A. The adult Drosophila posterior midgut is maintained by pluripotent stem cells. Nature 439, 470–474 (2006).
Biteau, B., Hochmuth, C. E. & Jasper, H. Maintaining tissue homeostasis: dynamic control of somatic stem cell activity. Cell Stem Cell 9, 402–411 (2011).
Jiang, H. et al. Cytokine/Jak/Stat signaling mediates regeneration and homeostasis in the Drosophila midgut. Cell 137, 1343–1355 (2009).
Biteau, B. & Jasper, H. EGF signaling regulates the proliferation of intestinal stem cells in Drosophila. Development 138, 1045–1055 (2011).
Xu, N. et al. EGFR, Wingless and JAK/STAT signaling cooperatively maintain Drosophila intestinal stem cells. Dev. Biol. 354, 31–43 (2011).
Buchon, N., Broderick, N. A., Kuraishi, T. & Lemaitre, B. Drosophila EGFR pathway coordinates stem cell proliferation and gut remodeling following infection. BMC Biol. 8, 152 (2010).
Jiang, H., Grenley, M. O., Bravo, M. J., Blumhagen, R. Z. & Edgar, B. A. EGFR/Ras/MAPK signaling mediates adult midgut epithelial homeostasis and regeneration in Drosophila. Cell Stem Cell 8, 84–95 (2011).
Buchon, N., Broderick, N. A., Chakrabarti, S. & Lemaitre, B. Invasive and indigenous microbiota impact intestinal stem cell activity through multiple pathways in Drosophila. Genes Dev. 23, 2333–2344 (2009).
Cronin, S. J. F. et al. Genome-wide RNAi screen identifies genes involved in intestinal pathogenic bacterial infection. Science 325, 340–343 (2009).
Cordero, J. B. & Sansom, O. J. Wnt signalling and its role in stem cell-driven intestinal regeneration and hyperplasia. Acta Physiol. 204, 137–143 (2012).
Li, H., Qi, Y. & Jasper, H. Dpp signaling determines regional stem cell identity in the regenerating adult Drosophila gastrointestinal tract. Cell Rep. 4, 10–18 (2013).
Li, Z., Zhang, Y., Han, L., Shi, L. & Lin, X. Trachea-derived dpp controls adult midgut homeostasis in Drosophila. Dev. Cell 24, 133–143 (2013).
Guo, Z., Driver, I. & Ohlstein, B. Injury-induced BMP signaling negatively regulates Drosophila midgut homeostasis. J. Cell Biol. 201, 945–961 (2013).
Tian, A. & Jiang, J. Intestinal epithelium-derived BMP controls stem cell self-renewal in Drosophila adult midgut. eLife 3, e01857 (2014).
Zhou, J. et al. Dpp/Gbb signaling is required for normal intestinal regeneration during infection. Dev. Biol. 399, 189–203 (2014).
Skoczek, D. A. et al. Luminal microbes promote monocyte–stem cell interactions across a healthy colonic epithelium. J. Immunol. 193, 439–451 (2014).
Pull, S. L., Doherty, J. M., Mills, J. C., Gordon, J. I. & Stappenbeck, T. S. Activated macrophages are an adaptive element of the colonic epithelial progenitor niche necessary for regenerative responses to injury. Proc. Natl Acad. Sci. USA 102, 99–104 (2005).
Malvin, N. P., Seno, H. & Stappenbeck, T. S. Colonic epithelial response to injury requires Myd88 signaling in myeloid cells. Mucosal Immunol. 5, 194–206 (2012).
Lemaitre, B. & Hoffmann, J. The host defense of Drosophila melanogaster. Annu. Rev. Immunol. 25, 697–743 (2007).
Babcock, D. T. et al. Circulating blood cells function as a surveillance system for damaged tissue in Drosophila larvae. Proc. Natl Acad. Sci. USA 105, 10017–10022 (2008).
Charroux, B. & Royet, J. Elimination of plasmatocytes by targeted apoptosis reveals their role in multiple aspects of the Drosophila immune response. Proc. Natl Acad. Sci. USA 106, 9797–9802 (2009).
Zaidman-Remy, A., Regan, J. C., Brandao, A. S. & Jacinto, A. The Drosophila larva as a tool to study gut-associated macrophages: PI3K regulates a discrete hemocyte population at the proventriculus. Dev. Comp. Immunol. 36, 638–647 (2012).
Tokusumi, T., Shoue, D. A., Tokusumi, Y., Stoller, J. R. & Schulz, R. A. New hemocyte-specific enhancer-reporter transgenes for the analysis of hematopoiesis in Drosophila. Genesis 47, 771–774 (2009).
Kurucz, E. et al. Nimrod, a putative phagocytosis receptor with EGF repeats in Drosophila plasmatocytes. Curr. Biol. 17, 649–654 (2007).
Amcheslavsky, A., Jiang, J. & Ip, Y. T. Tissue damage-induced intestinal stem cell division in Drosophila. Cell Stem Cell 4, 49–61 (2009).
Makhijani, K., Alexander, B., Tanaka, T., Rulifson, E. & Bruckner, K. The peripheral nervous system supports blood cell homing and survival in the Drosophila larva. Development 138, 5379–5391 (2011).
Lee, T. & Luo, L. Mosaic analysis with a repressible cell marker (MARCM) for Drosophila neural development. Trends Neurosci. 24, 251–254 (2001).
Zettervall, C. J. et al. A directed screen for genes involved in Drosophila blood cell activation. Proc. Natl Acad. Sci. USA 101, 14192–14197 (2004).
Clark, R. I., Woodcock, K. J., Geissmann, F., Trouillet, C. & Dionne, M. S. Multiple TGF-β superfamily signals modulate the adult Drosophila immune response. Curr. Biol. 21, 1672–1677 (2011).
Teleman, A. A. & Cohen, S. M. Dpp gradient formation in the Drosophila wing imaginal disc. Cell 103, 971–980 (2000).
Harrison, D. A. & Perrimon, N. Simple and efficient generation of marked clones in Drosophila. Curr. Biol. 3, 424–433 (1993).
Bardet, P. L. et al. A fluorescent reporter of caspase activity for live imaging. Proc. Natl Acad. Sci. USA 105, 13901–13905 (2008).
Hamaratoglu, F., de Lachapelle, A. M., Pyrowolakis, G., Bergmann, S. & Affolter, M. Dpp signaling activity requires Pentagone to scale with tissue size in the growing Drosophila wing imaginal disc. PLoS Biol. 9, e1001182 (2011).
Brummel, T. J. et al. Characterization and relationship of Dpp receptors encoded by the saxophone and thick veins genes in Drosophila. Cell 78, 251–261 (1994).
Henderson, K. D. & Andrew, D. J. Identification of a novel Drosophila SMAD on the X chromosome. Biochem. Biophys. Res. Commun. 252, 195–201 (1998).
Spokony, R. & White, K. Spokony Insertions (Flybase, 2013).
Zhu, C. C. et al. Drosophila Activin- and the Activin-like product Dawdle function redundantly to regulate proliferation in the larval brain. Development 135, 513–521 (2008).
Kim, M. J. & O’Connor, M. B. Anterograde activin signaling regulates postsynaptic membrane potential and GluRIIA/B abundance at the Drosophila neuromuscular junction. PLoS ONE 9, e107443 (2014).
Bangi, E. & Wharton, K. Dual function of the Drosophila Alk1/Alk2 ortholog Saxophone shapes the Bmp activity gradient in the wing imaginal disc. Development 133, 3295–3303 (2006).
Haerry, T. E., Khalsa, O., O’Connor, M. B. & Wharton, K. A. Synergistic signaling by two BMP ligands through the SAX and TKV receptors controls wing growth and patterning in Drosophila. Development 125, 3977–3987 (1998).
Biteau, B. et al. Lifespan extension by preserving proliferative homeostasis in Drosophila. PLoS Genet. 6, e1001159 (2010).
Guo, L., Karpac, J., Tran, S. L. & Jasper, H. PGRP-SC2 promotes gut immune homeostasis to limit commensal dysbiosis and extend lifespan. Cell 156, 109–122 (2014).
Rera, M., Clark, R. I. & Walker, D. W. Intestinal barrier dysfunction links metabolic and inflammatory markers of aging to death in Drosophila. Proc. Natl Acad. Sci. USA 109, 21528–21533 (2012).
Godwin, J. W., Pinto, A. R. & Rosenthal, N. A. Macrophages are required for adult salamander limb regeneration. Proc. Natl Acad. Sci. USA 110, 9415–9420 (2013).
Assoian, R. K. et al. Expression and secretion of type β transforming growth factor by activated human macrophages. Proc. Natl Acad. Sci. USA 84, 6020–6024 (1987).
Miyoshi, H., Ajima, R., Luo, C. T., Yamaguchi, T. P. & Stappenbeck, T. S. Wnt5a potentiates TGF-β signaling to promote colonic crypt regeneration after tissue injury. Science 338, 108–113 (2012).
Wakefield, L. M. & Hill, C. S. Beyond TGFβ: roles of other TGFβ superfamily members in cancer. Nat. Rev. Cancer 13, 328–341 (2013).
Peterson, A. J. & O’Connor, M. B. Strategies for exploring TGF-β signaling in Drosophila. Methods 68, 183–193 (2014).
Twombly, V. et al. Functional analysis of saxophone, the Drosophila gene encoding the BMP type I receptor ortholog of human ALK1/ACVRL1 and ACVR1/ALK2. Genetics 183, 563–579 (2009) 1SI-8SI
Bangi, E. & Wharton, K. Dpp and Gbb exhibit different effective ranges in the establishment of the BMP activity gradient critical for Drosophila wing patterning. Dev. Biol. 295, 178–193 (2006).
Haerry, T. E. The interaction between two TGF-β type I receptors plays important roles in ligand binding, SMAD activation, and gradient formation. Mech. Dev. 127, 358–370 (2010).
Brummel, T. et al. The Drosophila activin receptor baboon signals through dSmad2 and controls cell proliferation but not patterning during larval development. Genes Dev. 13, 98–111 (1999).
Wang, X., Harris, R. E., Bayston, L. J. & Ashe, H. L. Type IV collagens regulate BMP signalling in Drosophila. Nature 455, 72–77 (2008).
Li, C. Y., Guo, Z. & Wang, Z. TGFβ receptor saxophone non-autonomously regulates germline proliferation in a Smox/dSmad2-dependent manner in Drosophila testis. Dev. Biol. 309, 70–77 (2007).
Isogaya, K. et al. A Smad3 and TTF-1/NKX2-1 complex regulates Smad4-independent gene expression. Cell Res. 24, 994–1008 (2014).
Shaw, A. C., Goldstein, D. R. & Montgomery, R. R. Age-dependent dysregulation of innate immunity. Nat. Rev. Immunol. 13, 875–887 (2013).
Wang, G. C. & Casolaro, V. Immunologic changes in frail older adults. Transl. Med. UniSa 9, 1–6 (2014).
Khor, B., Gardet, A. & Xavier, R. J. Genetics and pathogenesis of inflammatory bowel disease. Nature 474, 307–317 (2011).
Wang, L., Zeng, X., Ryoo, H. D. & Jasper, H. Integration of UPRER and oxidative stress signaling in the control of intestinal stem cell proliferation. PLoS Genet. 10, e1004568 (2014).
Osterwalder, T., Yoon, K. S., White, B. H. & Keshishian, H. A conditional tissue-specific transgene expression system using inducible GAL4. Proc. Natl Acad. Sci. USA 98, 12596–12601 (2001).
Ni, J. Q. et al. A genome-scale shRNA resource for transgenic RNAi in Drosophila. Nat. Methods 8, 405–407 (2011).
McGuire, S. E., Mao, Z. & Davis, R. L. Spatiotemporal gene expression targeting with the TARGET and gene-switch systems in Drosophila. Sci. STKE 2004, pl6 (2004).
Acknowledgements
This work was financially supported by the National Institute on General Medical Sciences (R01 GM100196) and the National Eye Institute (R01 EY018177). We would like to thank J. Karpac for comments.
Author information
Authors and Affiliations
Contributions
A.A. and H.J. designed all experiments. A.A. generated transgenic animals and performed experiments on haemocytes, DPP, SAX and SMOX signalling, interactions of SAX/SMOX signalling with TKV/MAD, EGFR and JAK/STAT pathways and role of TKV expression in mitosis and on ageing, dysplasia and lifespan; H.L. validated specificity of TKV and SAX antibodies, performed experiments on TKV expression and on lineage tracing of MAD, MED and TKV mutant ISCs, and provided additional reagents for other experiments. A.A. and H.J. analysed the data and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
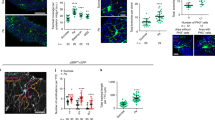
Supplementary Figure 3 Gut-associated hemocytes are required for ISC proliferation.
(A,B) Hemocyte-ablated larvae (A) and adults (B) are shown, generated by expressing the pro-apoptotic gene hid specifically in hemocytes using hmlΔ::Gal4;UAS::GFP. GFP-positive hemocyte clumps are indicated by red arrowheads. (C) hml promoter expression in the whole adult gut is shown using hmlΔ::Gal4 (i). Variable number of extra-epithelial GFP-positive hemocytes can be detected in the proventriculous (ii; note that hemocytes (yellow arrowheads) are not part of the intestinal epithelium (white arrowheads), sometimes attached to the epithelial surface in midgut (iii), or hindgut regions (iv) but are not part of the epithelium that contains Delta-positive and esg::Gal4 expressing ISCs (v, vi). (D,E) hmlΔ::Gal4;UAS::GFP+ hemocytes attaching the abdominal body wall (D) also express the phagocyte marker NimC1 (E). Ovaries and fat body are hmlΔ::Gal4- and NimC1- (E). (F) Hemocyte-deficient flies rapidly succumb while both HDD and wild-type flies survive systemically disseminated P. entomophila. (G) Intestine in hemoless flies is normal in size and structure, and contain a normal distribution of cells (βCatenin/Armadillo labels cell boundaries and is highly expressed in ISC/EB nests; Prospero in enteroendocrine cells, and Delta in ISCs). (H) Intestinal cell composition. Absence of hemocytes does not change the composition of cells in the midgut of young animals. However, an increased number of Delta-positive ISCs observed in old wild-type animals is reduced in hemoless flies. (I) Representative images showing that intact Delta+ ISCs can be detected in the posterior midguts of hemocless flies at 12 h post-Ecc15 infection. Error bars indicate s.e.m. (H: n = 10 and I: n = 8 flies tested in a single experiment, P values taken from Student Ttest; and experiment was replicated 3 (in panel H) and 2 (in panel I) times. Survival curves shown in panel F were compared using Prism software (n = 20 flies tested in one experiment, p values were taken from LogRank test, and experiment was repeated 3 times. Single representative images from 10 flies used in a single experiment are shown in panels A–E and G while experiment was reproduced 3 (in panels A,B,D–I and G) or 2 (in panel C) times. n.s. = non-significant,∗∗p < 0.01,∗∗∗p < 0.001.
Supplementary Figure 4 Hemocytes recruited to stressed guts represent a post mitotic hematopoietic population.
(A) PQ-induced ISC proliferation when hemocytes were ablated at 2 days of age by expressing hid with hmlΔ::Gal4, UAS::GFP, tub::Gal80ts for 4 days. Hemocyte ablation was monitored visually in intact adults (not shown). Flies were exposed to Paraquat (PQ in 5% sucrose) or Mock (5% sucrose) for 16 h at 25 °C. (B) Hemocytes attached to the external surface of midgut regions a and b (identified by how::Gal4, UAS::GFP; see Fig. 2b), following 2 days of P. entomophila infection. Quantification of eater::DsRed+ cells per gut-associated hemocyte clump 2 days after P. entomophila or 4 h after Ecc15 infection; M: mock. (C) 5966::GS, UAS::GFP flies co-expressing eater::DsRed fed RU846 food for 2 days followed by oral infection with Ecc15 for 4 h. (D) Circulating hemocytes change their size and shape at 4 h post-Ecc15 infection. F-actin detected by Phalloidin (red). (E,F) MARCM competent flies co-expressing eater::DsRed (see Supplementary Table 1 for exact genotype) subjected to Ecc15 challenge and simultaneously heat shocked. No GFP+ hemocytes were observed at 12 h after Ecc15 infection (Analysis I) or at 5 days post heat shock (with or without a second Ecc15 challenge: Analysis II) in any tissue (E; and not shown). GFP+ ISC-derived clones can be observed within the intestinal epithelium (F). (G) Expression pattern of dve::Gal4, UAS::GFP in midgut. PV: proventriculus, AM: anterior midgut, PM (b–d): posterior midgut regions b–d, HG: hindgut; Cut marks Copper Cells in middle midgut (compare18), here designated as region ‘a’ of the midgut. Error bars indicate either s.e.m. (A: n = 12 flies) or range (B: for mock/P.e.: n = 22 flies, for mock/Ecc15: n = 32 flies; where boxes show 25–75% percentile and horizontal bar within each box is population median) calculated from single experiment. P values from Student’s Ttest, reproduced in 3 independent experiments. Single representative image from 15 flies is shown in B–D,F and G, experiment was repeated 3 (in panels B,C and G) and 2 (in panel D–F) times. ∗∗∗P < 0.001.
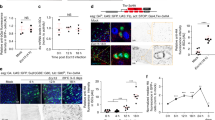
Supplementary Figure 5 Hemocyte-derive Dpp is required for ISC proliferation.
(A) Hemocyte-specific RNAi screen assessing ISC activity upon PQ treatment. (B) Knockdown of Dpp using hmlΔ::GS. RU486 fed for 2 days followed by 8 h Ecc15 challenge. (C) ISC proliferation at 8 h post-Ecc15 in animals with knockdown of Dpp in hemocytes (hmlΔ::G4 and He::Gal4), hindgut (byn::Gal4) or proventriculus (Cardia::Gal4). (D) ISC proliferation upon Paraquat (PQ) feeding after Dpp knock down in ECs (NP1::Gal4), ISCs and EBs (esg::Gal4), trachea (Btl::Gal4) or visceral muscle (how::Gal4). (E) qRT-PCR to assess effectiveness of DppRNAi lines in 10–15 day old adult carcass (containing hemocytes) using hmlΔ::Gal4, or in whole guts when using how::G4ts (29 °C 48 h). (F) RU486-sensitive expression of UAS::mCD8-GFP in hemocytes and lymph gland of hmlΔ::GS larvae (no expression in adult gut epithelium). (G) GFP-positive, Dpp-deficient hemocytes in larvae and adults. Circulating hemocytes in adult hemolymph collected by Nanoinject II and counted using hemocytometer. (H) Dpp-deficient hemocytes attach to the intestinal epithelium during Ecc15 challenge. (I) Percentage of GFP-positive hemocytes 16 h post-PQ treatment in Dpp::Gal4, UAS::GFP flies. (J) Dpp transcript in hemocytes upon infection with Ecc15 (30 min after challenge). (K) Dpp-GFP from hemocytes (2 day feeding RU486) accumulates on ISCs (4 h Ecc15). (L) Clones generated by FRT-mediated reconstitution of split actin::LacZ in wild-type or hemoless (hml::Gal4, UAS::Rpr) backgrounds. (M) Assessment of apoptosis by Apoliner (UAS::mcd8RFP::nlsGFP) expressed in ISC progeny (5966::GS). No apoptotic cells (nuclear GFP) are observed in hml::DppRNAi flies. Apoptotic ECs can be observed during Ecc15 infection. (N) Number of hemocytes retrieved from hemolymph at indicated ages. (O) PQ-induced recruitment of transplanted hemocytes to the gut of hemoless flies (donor: hmlΔ::Gal4, UAS::GFP). Error bars indicate s.e.m. (A: n’s are indicated, composite of two (first graph) or three (second graph) experiments; B,E: n = 10; G: n = 5 (each sample represents a cohort of 20 flies); C,D: n = 10; I,J: n = 10; N: n = 7) or range (O: n = 9; boxes: 25–75% percentile. horizontal bar median value), Student T-test. All experiments, except A, replicated three times independently. Representative image (n = 9) in F–I and K–M; experiment repeated 3 times. n.s. = non-significant,∗∗∗P < 0.001.
Supplementary Figure 6 Hemocyte-derived Dpp requires Jak/Stat and EGFR pathways to induce proliferation.
(A–C) Transcription of Jak/Stat pathway activating ligand upd3 (A), expression of Jak/Stat activity reporter STAT::GFP (B), and EGFR ligand reporter Vein::LacZ (C: arrowheads) are normally induced in the intestine of HDD (A,C) and hemoless (B) flies during Ecc15 infection. (D) ISC proliferation induced upon upd2 overexpression in ECs is significantly reduced when Dpp expression is simultaneously blocked in hemocytes using an hmlΔ-DppRNAi lines that does not use Gal4/UAS system (see Methods). (E,F) Expression of UAS::Dpp was induced in adult hemocytes under the control of hmlΔ::Gal4 driver (which co-expressed tub-Gal80ts) by shifting 3 days old adult flies from 18 °C to 29 °C for indicated time intervals. Short-term expression of hemocyte-derived Dpp (that is, for 8 or 48 h) did not induce mitotic response in ISCs (E), while higher ISC proliferation was observed upon a long-term expression of Dpp in hemocytes for 13 days period. Over-expression of Dpp in visceral muscle (How::Gal4) for 8 or 24 h does also not induce ISC proliferation. Error bars indicate s.e.m. (n > 7), P values from Student T test. (G,H) HDD flies exhibit normal feeding in the absence of stress (assessed by the CAFÉ assay; G) or when fed on Ecc15 infection solution mixed with a blue dye for 90 min, following 2 h of starvation period (H; note that digestive tracts are full of blue dye). Error bars indicate s.e.m. (A: n = 4; D–F: n = 10; and G: n = 9 flies were used in one experiment), P values from Student T test, while results were reproduced in 2 independent experiments. Panels B (n > 9), C (n > 8) and H (n > 15) show single representative images from 9 (panel B), 8 (panel C) and 15 (H) flies, and each experiment was replicated twice. n.s. = non-significant,∗P < 0.05,∗∗P < 0.01,∗∗∗P < 0.001.
Supplementary Figure 7 Hemocyte-derived Dpp induces ISC proliferation in all gut regions, independent of Mad.
(A) Reduced ISC proliferative response in HDD flies upon Ecc15 infection is observed in the entire length of the Drosophila adult midgut: anterior midgut (AM), Copper Cell Region (region ‘a’) and posterior midgut (regions ‘b–d’). (B) Induction of local Dpp expression in the visceral muscle at region ‘c’ is observed only after 16 h post-Ecc15 infection. (C) Mad is not phosphorylated in ISCs of region ‘c’ of the PM 4 h post Ecc15 infection, but phosphorylation can be detected at 16 h after challenge (AC): arrowheads. Delta as well as YFP-positive cells are ISCs. (D) Ubiquitous knockdown of Dpp, but not of dActivin or dawdle, using Mifepristone (RU486) drug sensitive ubiquitously expressed driver tub-GS decreases ISC proliferative response 4 h post-Ecc15 challenge. (E) Knock down of Smox using ISC-specific 5961::GS significantly reduces the expression of dad::nGFP specifically in ISCs, but not in other intestinal cells, in region c of the posterior midgut following 4 h of Ecc15 challenge (compare to Fig. 4e). Error bars indicate s.e.m. (A: n = 10 and D: n = 7 flies from one experiment), P values taken from Student T test, while each experiment was repeated 3 times. One representative image is shown from 13 (in panel B), 7 (in panel C) and 10 (panel F) flies used in a single experiment, while each experiment was repeated twice (B) or three times (C,F). n.s. = non-significant.
Supplementary Figure 8 Hemocyte-derived Dpp signals through Sax/Smox to induce ISC proliferation.
(A) ISC proliferation at 4 h of Ecc15 challenge. Gbb is knocked in adults in ECs (NP1::Gal4ts) or hemocytes (hmlΔ::Gal4ts). (B) Mad phosphorylation is absent in Tkv-, but not Sax-, mutant ISCs in MARCM clones in region ‘c’ of PM 24 h post-Ecc15 challenge (arrowheads). (C) Over-expression of a constitutively active TkvQD in ISCs at region ‘c’ is sufficient to phosphorylate Mad in the absence of injury. (D) Representative images showing MARCM clones generated under normal conditions by mad12, mad1−2, tkv04415, Tkva12, med13, Sax4 and SmoxMB388 mutant ISCs, and following Ecc15 oral challenge by tkv04415 and mad12 mutant ISCs alone combined with ISC-specific Sax or Smox knockdown. (E) Smox knock down in ISCs suppresses the growth of ISC tumours in Notch loss-of-function conditions, which are formed by the accumulation of symmetrically dividing Delta-positive ISCs, a phenotype observed when NotchRNAi is expressed under the control of ISC/EB specific driver esg::Gal4. (F) ISC over-proliferation induced by expressing EGFR-gain-of-function (EGFR-GOF) or constitutively active Hop (HopTumL) is rescued by simultaneous Smox knockdown. (G) Number of gut-associated eater::DsRed+ hemocytes does not increase upon EGFR-GOF over-expression. Error bars indicate either s.e.m. (A: n = 14 and F: n = 10 flies) or range (G: n = 7 flies; where boxes show 25–75% percentile and horizontal bar within each box is population median) from one experiment, P values from Student T test; while each experiment was repeated twice. One representative image from 7 (panels B,C and G) and 10 (panel E) flies used in a single experiment is shown, while experiment was repeated 3 (panels B,C,E) or 2 (panel G) times. Panel D shows one representative images for each data set quantified in Fig. 5d, e (see legends of Fig. 5d, e for n, P values and repetitions).
Supplementary Figure 9 Sax does not induce Tkv expression.
(A) Sax detection in ISCs is lost upon RNAi knockdown using ISC/EB specific driver esg-Gal4, while an excessive staining is observed upon Sax overexpression within these cells (arrowheads), thus validating the specificity of the antibody used in this study. (B) Low expression of Tkv is detected in ISCs during basal conditions. TkvQD, when over-expressed in ISCs and EBs under the control of esg-Gal4, can be readily detected in these cells (arrows), confirming the specificity of the antibody used. (C) Sax knockdown in ISCs does not prevent late Tkv induction in ISCs following Ecc15 challenge (arrowheads). One representative image from 7 flies used in single experiment is shown in all panels, while each experiment was repeated twice.
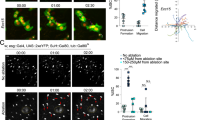
Supplementary Figure 10 Hemocyte-derived Dpp promotes intestinal dysplasia by inducing Smox nuclear localization.
(A) Large numbers of hmlΔ::Gal4, UAS::GFP+ hemocytes (both as single cells or clumps) can be found attached to the intestine of young flies 8 h after Ecc15 challenge as well as in 30 day old untreated flies. (B–D) Images show hemocyte-derived Dpp dependent activation of BMP signalling pathway activation (note dad::nGFP reporter activity) in region c of posterior midgut (B), Smox nuclear localization (C) and development of intestinal dysplasia (D; note accumulation of Delta-positive cells). (E) Aged related overactivation of ISC proliferation, identified by the frequency of phospho histone H3+ (pH3+) cells per gut, is significantly reduced when 24 days old 5961::GS flies co-expressing UAS::Tkv transgene were shifted to RU846 drug containing food before observation at 30 days of age, thus, indicating that a relatively acute overexpression (for 6 days) of wild-type Tkv construct in ISCs can inhibit over activation of ISCs in aging animals. (F) Results from lifespan experiments are showing that where hemoless are significantly short-live, HDD flies live as long as their wild-type counter parts. Each survival curve represents a composite of total number of animals of a particular genotype tested (also see methods: lifespan experiments). Error bars indicate s.e.m. (E: n = 14 flies used in one experiment), p values taken from Student T test, while results were reproduced twice. One representative image from 15 (panel A) or 7 (panels B–D) flies used in one experiment is shown, and each experiment was repeated two times.
Supplementary information
Supplementary Information
Supplementary Information (PDF 2080 kb)
Rights and permissions
About this article
Cite this article
Ayyaz, A., Li, H. & Jasper, H. Haemocytes control stem cell activity in the Drosophila intestine. Nat Cell Biol 17, 736–748 (2015). https://doi.org/10.1038/ncb3174
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb3174
This article is cited by
-
Drosophila activins adapt gut size to food intake and promote regenerative growth
Nature Communications (2024)
-
Targeting the “hallmarks of aging” to slow aging and treat age-related disease: fact or fiction?
Molecular Psychiatry (2023)
-
Drosophila ML-DmD17-c3 cells respond robustly to Dpp and exhibit complex transcriptional feedback on BMP signaling components
BMC Developmental Biology (2019)
-
AWD regulates timed activation of BMP signaling in intestinal stem cells to maintain tissue homeostasis
Nature Communications (2019)
-
Loss of a proteostatic checkpoint in intestinal stem cells contributes to age-related epithelial dysfunction
Nature Communications (2019)