Abstract
Germ cells divide and differentiate in a unique local microenvironment under the control of somatic cells. Signals released in this niche instruct oocyte reentry into the meiotic cell cycle. Once initiated, the progression through meiosis and the associated programme of maternal messenger RNA translation are thought to be cell autonomous. Here we show that translation of a subset of maternal mRNAs critical for embryo development is under the control of somatic cell inputs. Translation of specific maternal transcripts increases in oocytes cultured in association with somatic cells and is sensitive to EGF-like growth factors that act only on the somatic compartment. In mice deficient in amphiregulin, decreased fecundity and oocyte developmental competence is associated with defective translation of a subset of maternal mRNAs. These somatic cell signals that affect translation require activation of the PI(3)K–AKT–mTOR pathway. Thus, mRNA translation depends on somatic cell cues that are essential to reprogramme the oocyte for embryo development.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Accession codes
References
Spradling, A., Fuller, M. T., Braun, R. E. & Yoshida, S. Germline stem cells. Cold Spring Harb. Perspect. Biol. 3, a002642 (2011).
Yoshida, S. Spermatogenic stem cell system in the mouse testis. Cold Spring Harb. Symp. Quant. Biol. 73, 25–32 (2008).
Matzuk, M. M., Burns, K. H., Viveiros, M. M. & Eppig, J. J. Intercellular communication in the mammalian ovary: oocytes carry the conversation. Science 296, 2178–2180 (2002).
Gosden, R. & Lee, B. Portrait of an oocyte: our obscure origin. J. Clin. Invest. 120, 973–983 (2010).
Chian, R. C., Lim, J. H. & Tan, S. L. State of the art in in-vitro oocyte maturation. Curr. Opin. Obstet. Gynecol. 16, 211–219 (2004).
Chang, H. C., Liu, H., Zhang, J., Grifo, J. & Krey, L.C. Developmental incompetency of denuded mouse oocytes undergoing maturation in vitro is ooplasmic in nature and is associated with aberrant Oct-4 expression. Human Reprod. 20, 1958–1968 (2005).
Luciano, A. M. et al. Developmental capability of denuded bovine oocyte in a co-culture system with intact cumulus-oocyte complexes: role of cumulus cells, cyclic adenosine 3’,5’-monophosphate, and glutathione. Mol. Reproduct. Dev. 71, 389–397 (2005).
Eppig, J. J. Coordination of nuclear and cytoplasmic oocyte maturation in eutherian mammals. Reprod. Fertil. Dev. 8, 485–489 (1996).
Park, J. Y. et al. EGF-like growth factors as mediators of LH action in the ovulatory follicle. Science 303, 682–684 (2004).
Shimada, M., Hernandez-Gonzalez, I., Gonzalez-Robayna, I. & Richards, J. S. Paracrine and autocrine regulation of epidermal growth factor-like factors in cumulus oocyte complexes and granulosa cells: key roles for prostaglandin synthase 2 and progesterone receptor. Mol. Endocrinol. 20, 1352–1365 (2006).
Hsieh, M. et al. Luteinizing hormone-dependent activation of the epidermalgrowth factor network is essential for ovulation. Mol. Cell Biol. 27, 1914–1924 (2007).
Panigone, S., Hsieh, M., Fu, M., Persani, L. & Conti, M. Luteinizing hormone signaling in preovulatory follicles involves early activation of the epidermal growth factor receptor pathway. Mol. Endocrinol. 22, 924–936 (2008).
Hsieh, M., Thao, K. & Conti, M. Genetic dissection of epidermal growth factor receptor signaling during luteinizing hormone-induced oocyte maturation. PLoS ONE 6, e21574 (2011).
Richter, J. D. CPEB: a life in translation. Trends Biochem. Sci. 32, 279–285 (2007).
Gruss, O. J. et al. Chromosome-induced microtubule assembly mediated by TPX2 is required for spindle formation in HeLa cells. Nat. Cell Biol. 4, 871–879 (2002).
De Luca, M., Lavia, P. & Guarguaglini, G. A functional interplay between Aurora-A, Plk1 and TPX2 at spindle poles: Plk1 controls centrosomal localization of Aurora-A and TPX2 spindle association. Cell Cycle 5, 296–303 (2006).
Kufer, T. A. et al. Human TPX2 is required for targeting Aurora-A kinase to the spindle. J. Cell Biol. 158, 617–623 (2002).
Chang, H. et al. The TPX2 gene is a promising diagnostic and therapeutic target for cervical cancer. Oncol. Rep. 27, 1353–1359 (2012).
Ma, Y. et al. Expression of targeting protein for xklp2 associated with both malignant transformation of respiratory epithelium and progression of squamous cell lung cancer. Clin. Cancer Res. 12, 1121–1127 (2006).
Shigeishi, H. et al. Expression of TPX2 in salivary gland carcinomas. Oncol. Rep. 21, 341–344 (2009).
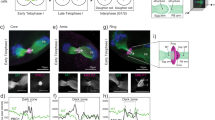
Brunet, S. et al. Meiotic regulation of TPX2 protein levels governs cell cycle progression in mouse oocytes. PLoS ONE 3, e3338 (2008).
Chen, J. et al. Genome-wide analysis of translation reveals a critical role for deleted in azoospermia-like (Dazl) at the oocyte-to-zygote transition. Genes Dev. 25, 755–766 (2011).
Brook, M., Smith, J. W. & Gray, N. K. The DAZL and PABP families: RNA-binding proteins with interrelated roles in translational control in oocytes. Reproduction 137, 595–617 (2009).
Conti, M., Hsieh, M., Park, J. Y. & Su, Y. Q. Role of the epidermal growth factor network in ovarian follicles. Mol. Endocrinol. 20, 715–723 (2006).
Fan, H. Y. et al. Selective expression of KrasG12D in granulosa cells of the mouse ovary causes defects in follicle development and ovulation. Development 135, 2127–2137 (2008).
De La Fuente, R., O’Brien, M. J. & Eppig, J. J. Epidermal growth factor enhances preimplantation developmental competence of maturing mouse oocytes. Hum. Reprod. 14, 3060–3068 (1999).
Prochazka, R., Kalab, P. & Nagyova, E. Epidermal growth factor-receptor tyrosine kinase activity regulates expansion of porcine oocyte-cumulus cell complexes in vitro. Biol. Reprod. 68, 797–803 (2003).
Richani, D., Ritter, L. J., Thompson, J. G. & Gilchrist, R. B. Mode of oocyte maturation affects EGF-like peptide function and oocyte competence. Mol. Hum. Rreprod. 19, 500–509 (2013).
Han, S. J. et al. Protein kinase B/Akt phosphorylation of PDE3A and its role in mammalian oocyte maturation. EMBO J. 25, 5716–5725 (2006).
Kalous, J. et al. PKB/AKT is involved in resumption of meiosis in mouse oocytes. Biol. Cell 98, 111–123 (2006).
Grzmil, M. & Hemmings, B. A. Translation regulation as a therapeutic target in cancer. Cancer Res. 72, 3891–3900 (2012).
Jagarlamudi, K. et al. Oocyte-specific deletion of Pten in mice reveals a stage-specific function of PTEN/PI3K signaling in oocytes in controlling follicular activation. PLoS ONE 4, e6186 (2009).
Busser, B., Sancey, L., Brambilla, E., Coll, J. L. & Hurbin, A. Themultiple of amphiregulin in human cancer. Biochim. Biophys. Acta 1816, 119–131 (2011).
Hynes, N. E. & Lane, H. A. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat. Rev. Cancer 5, 341–354 (2005).
Ma, X. M. & Blenis, J. Molecular mechanisms of mTOR-mediated translational control. Nat. Rev. Mol. Cell Biol. 10, 307–318 (2009).
Inoki, K., Kim, J. & Guan, K. L. AMPK and mTOR in cellular energy homeostasis and drug targets. Annu. Rev. Pharmacol. Toxicol. 52, 381–400 (2012).
Magnuson, B., Ekim, B. & Fingar, D. C. Regulation and function of ribosomalprotein S6 kinase (S6K) within mTOR signalling networks. Biochem. J. 441, 1–21 (2012).
Ruggero, D. & Sonenberg, N. The Akt of translational control. Oncogene 24, 7426–7434 (2005).
Meyuhas, O. Synthesis of the translational apparatus is regulated at the translational level. Eur. J. Biochem. 267, 6321–6330 (2000).
Hsieh, A. C. et al. The translational landscape of mTOR signalling steers cancer initiation and metastasis. Nature 485, 55–61 (2012).
Barna, M. et al. Suppression of Myc oncogenic activity by ribosomal protein haploinsufficiency. Nature 456, 971–975 (2008).
Su, Y. Q. et al. Selective degradation of transcripts during meiotic maturation of mouse oocytes. Dev. Biol. 302, 104–117 (2007).
Irizarry, R. A. et al. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics 4, 249–264 (2003).
Dai, M. et al. Evolving gene/transcriptdefinitions significantly alter the interpretation of GeneChip data. Nucleic Acids Res. 33, e175 (2005).
Huang, D. W., Sherman, B. T. & Lempicki, R. A. Systematic and integrative analysis of large gene lists using DAVID Bioinformatics Resources. Nat. Protoc. 4, 44–57 (2009).
Huang, D. W., Sherman, B. T. & Lempicki, R. A. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists.. Nucleic Acids Res. 37, 1–13 (2009).
P., Machanick & Bailey, T. L. MEME-ChIP: motif analysis of large DNA datasets. Bioinformatics 2712, 1696–1697 (2011.).
Crooks, G. E., Hon, G. & Chandonia, J. M. Brenner SE WebLogo: a sequence logo generator. Genome Res. 14, 1188–1190 (200).
Acknowledgements
We thank D. Laird, D. Ruggero, T. Nystul and R. Belloch at UCSF for advice during the studies and for critical reading of the manuscript, and A. Susor for assisting with the oocyte confocal microscopy analysis. This work was supported by Eunice Kennedy Shriver National Institute of Child Health and Human Development/NIH cooperative agreement 1U54HD055764-06, as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research, and RO1-GM097165 to M.C.
Author information
Authors and Affiliations
Contributions
J.C. developed the CEO translation assay and carried out the microarray experiments and some of the AKT assays; S.T. carried out the characterization of the Areg null phenotype; F.X. contributed with western blot studies and oocyte isolation for microinjection; C-J.L. helped with immunostaining experiments and the microinjections in cumulus oocyte complexes; H.C. carried out the experiments on protein secretion and contributed to the writing of the manuscript. F.F. carried out microinjections in CEOs; K.H. contributed with the preparation of the translational luciferase reporters; C.O. and J.S.S. carried out the bioinformatic analysis of the microarray data. M.I.C. advised on data analysis and discussed results; M.R-S. provided reagents and constructs and advised in the interpretation of the data; M.C. conceived the project, designed the experiments, analysed the data and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 AREG-dependent stimulation of reporter translation is absent when meiotic reentry is prevented.
A. Cumulus enclosed oocytes were injected with TPX2 3’UTR Renilla luciferase reporter and cultured overnight in medium that enables maturation to MII or in medium that prevents maturation (2 μM milrinone) with or without AREG. At the end of the incubation oocytes were freed of surrounding cumulus cells and luciferase activity was measured in extracts from oocytes. The data are mean ± SEM of three to four independent experiments. *p<0.05 vs MII, **p<0.01 vs MII+AREG. B. Cumulus enclosed oocytes were injected with TPX2 3’UTR Renilla luciferase reporter (RLuc) and polyadenylated firefly reporter (FLuc) and cultured overnight as described above. Quantitative RT-PCR for RLuc and FLuc was performed from oocyte extracts. Exposure to Milrinone or AREG did not change the stability of the reporter. No significant differences between groups.
Supplementary Figure 2 AREG-dependent stimulation of reporter translation is absent when 3’UTR is truncated.
A. Diagram of the Renilla luciferase constructs injected to oocytes. B. Cumulus enclosed oocytes were injected with TPX2 3’UTR (1-1630) or TPX2 truncated 3’UTR Renilla luciferase reporter and cultured overnight in medium that enables maturation to MII with or without AREG/EGF or in medium that prevents maturation (2 μM milrinone). At the end of the incubation oocytes were freed of surrounding cumulus cells and luciferase activity was measured in extracts from oocytes. The data are mean ± SEM of three independent experiments. *p<0.05 vs control.
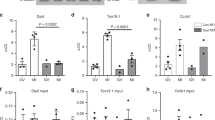
Supplementary Figure 3 Enrichment in 3’UTR motifs in transcripts deregulated in the Areg-/- mice.
Unique 3’UTR DNA sequence for all differentially translated transcripts (Supplemental Tables 1 and 2) was downloaded from the mm9 assembly of the UCSC Genome Browser. The sequences were scanned for motifs with MEME-ChIP (ref. 47), searching only the given strand for any number of repetitions and allowing for motifs of length between 6 and 30 bases. Only motifs with evalues less than 0.01 are reported. For each motif discovered by MEME-ChIP, fasta sequences were downloaded and converted to RNA sequence, and the analogous RNA motif sequence logos were generated using WebLogo 3 48. The motif reported in the top panel corresponds to the motif recognized by the Drosophila Hrb87F or Hrb98DE protein. These proteins are homologous to the mammalian A/B-type HnRNP proteins involved in transport and stabilization of mRNAs.
Supplementary Figure 4 Phosphorylation of AKT (Ser 473) in oocytes is dependent on the presence of somatic cells.
A. Representative time course of AKT Ser 473 phosphorylation in oocytes cultured as cumulus enclosed oocytes (CEOs). CEOs were incubated in medium with AREG and 2 μM milrinone to prevent maturation. At each time oocytes were freed of surrounding cumulus and used for Western blot analysis. Total AKT was used as loading control. A representative experiment of the three performed is reported. B. Representative Western blots of phosphorylated AKT in oocytes cultured as CEO or denuded as indicated times with or without AREG. Total AKT was used as loading control.
Supplementary Figure 5 In situ detection of AKT phosphorylation in oocytes stimulated with AREG when in complex with cumulus cells.
A. CEOs were cultured with or without AREG for 150 min; at the end of the incubation, oocytes were freed of cumulus cells and stained for phospho-AKT (green). B. Quantification of the intensity of the phospho-AKT from different experiments (mean ± SEM; n = 3). *p<0.05 vs CEO. The scale bar corresponds to 10 μm.
Supplementary Figure 6 mTOR inhibitors block the reporter translation stimulated by AREG.
A. Representative Western blot of phosphorylation of ribosomal protein S6 (rpS6) in oocytes cultured as CEO in medium containing 50 nM of rapamycin (mTOR inhibitor) or 100nM of INK128 (selective TORC1/2 inhibitor) for 150min. Tubulin was used as loading control. B. Quantification of the intensity of the phospho-rpS6 immunoreactive band from different experiments (mean ± SEM; n = 3) *P<0.05 vs AREG.
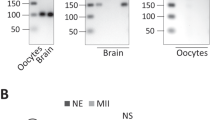
Supplementary Figure 7 Uncropped images of the Western blots included in the main text showing the molecular weight markers.
Dashed boxes indicate the portion of the gel included in the figure. The membrane used to generate Fig 1A, 2D and 2E were cut into two sections and developed with different antibodies. The black dotted line indicates the position of the cut. After development, the two sections of the blot were realigned and exposed together.
Supplementary information
Supplementary Information
Supplementary Information (PDF 1164 kb)
Supplementary Table 1
Supplementary Information (XLSX 2035 kb)
Supplementary Table 2
Supplementary Information (XLSX 2170 kb)
Supplementary Table 3
Supplementary Information (XLSX 32 kb)
Supplementary Table 4
Supplementary Information (XLSX 14 kb)
Rights and permissions
About this article
Cite this article
Chen, J., Torcia, S., Xie, F. et al. Somatic cells regulate maternal mRNA translation and developmental competence of mouse oocytes. Nat Cell Biol 15, 1415–1423 (2013). https://doi.org/10.1038/ncb2873
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb2873
This article is cited by
-
Decreased HAT1 expression in granulosa cells disturbs oocyte meiosis during mouse ovarian aging
Reproductive Biology and Endocrinology (2023)
-
CPEB1-dependent disruption of the mRNA translation program in oocytes during maternal aging
Nature Communications (2023)
-
The role of amphiregulin in ovarian function and disease
Cellular and Molecular Life Sciences (2023)
-
SphK-produced S1P in somatic cells is indispensable for LH-EGFR signaling-induced mouse oocyte maturation
Cell Death & Disease (2022)
-
Inhibiting bridge integrator 2 phosphorylation leads to improved oocyte quality, ovarian health and fertility in aging and after chemotherapy in mice
Nature Aging (2021)