Abstract
Adult differentiated cells can be reprogrammed into pluripotent stem cells or lineage-restricted proliferating precursors in culture; however, this has not been demonstrated in vivo. Here, we show that the single transcription factor SOX2 is sufficient to reprogram resident astrocytes into proliferative neuroblasts in the adult mouse brain. These induced adult neuroblasts (iANBs) persist for months and can be generated even in aged brains. When supplied with BDNF and noggin or when the mice are treated with a histone deacetylase inhibitor, iANBs develop into electrophysiologically mature neurons, which functionally integrate into the local neural network. Our results demonstrate that adult astrocytes exhibit remarkable plasticity in vivo, a feature that might have important implications in regeneration of the central nervous system using endogenous patient-specific glial cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Zhao, C., Deng, W. & Gage, F. H. Mechanisms and functional implications of adult neurogenesis. Cell 132, 645–660 (2008).
Kriegstein, A. & Alvarez-Buylla, A. The glial nature of embryonic and adult neural stem cells. Annu. Rev. Neurosci. 32, 149–184 (2009).
Ma, D. K., Bonaguidi, M. A., Ming, G. L. & Song, H. Adult neural stem cells in the mammalian central nervous system. Cell Res. 19, 672–682 (2009).
Hsieh, J. Orchestrating transcriptional control of adult neurogenesis. Genes Dev. 26, 1010–1021 (2012).
Kempermann, G., Jessberger, S., Steiner, B. & Kronenberg, G. Milestones of neuronal development in the adult hippocampus. Trends Neurosci. 27, 447–452 (2004).
Lugert, S. et al. Homeostatic neurogenesis in the adult hippocampus does not involve amplification of Ascl1(high) intermediate progenitors. Nat. Commun. 3, 670 (2012).
Arvidsson, A., Collin, T., Kirik, D., Kokaia, Z. & Lindvall, O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat. Med. 8, 963–970 (2002).
Magavi, S. S., Leavitt, B. R. & Macklis, J. D. Induction of neurogenesis in the neocortex of adult mice. Nature 405, 951–955 (2000).
Jablonska, B. et al. Chordin-induced lineage plasticity of adult SVZ neuroblasts after demyelination. Nat. Neurosci. 13, 541–550 (2010).
Takahashi, K. & Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676 (2006).
Han, D. W. et al. Direct reprogramming of fibroblasts into neural stem cells by defined factors. Cell Stem Cell 10, 465–472 (2012).
Kim, J. et al. Direct reprogramming of mouse fibroblasts to neural progenitors. Proc. Natl Acad. Sci. USA 108, 7838–7843 (2011).
Ring, K. L. et al. Direct reprogramming of mouse and human fibroblasts into multipotent neural stem cells with a single factor. Cell Stem Cell 11, 100–109 (2012).
Thier, M. et al. Direct conversion of fibroblasts into stably expandable neural stem cells. Cell Stem Cell 10, 473–479 (2012).
Lujan, E., Chanda, S., Ahlenius, H., Sudhof, T. C. & Wernig, M. Direct conversion of mouse fibroblasts to self-renewing, tripotent neural precursor cells. Proc. Natl Acad. Sci. USA 109, 2527–2532 (2012).
Ambasudhan, R. et al. Direct reprogramming of adult human fibroblasts to functional neurons under defined conditions. Cell Stem Cell 9, 113–118 (2011).
Caiazzo, M. et al. Direct generation of functional dopaminergic neurons from mouse and human fibroblasts. Nature 476, 224–227 (2011).
Heinrich, C. et al. Directing astroglia from the cerebral cortex into subtype specific functional neurons. PLoS Biol. 8, e1000373 (2010).
Heins, N. et al. Glial cells generate neurons: the role of the transcription factor Pax6. Nat. Neurosci. 5, 308–315 (2002).
Pfisterer, U. et al. Direct conversion of human fibroblasts to dopaminergic neurons. Proc. Natl Acad. Sci. USA 108, 10343–10348 (2011).
Vierbuchen, T. et al. Direct conversion of fibroblasts to functional neurons by defined factors. Nature 463, 1035–1041 (2010).
Karow, M. et al. Reprogramming of pericyte-derived cells of the adult human brain into induced neuronal cells. Cell Stem Cell 11, 471–476 (2012).
Zhou, Q., Brown, J., Kanarek, A., Rajagopal, J. & Melton, D. A. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature 455, 627–632 (2008).
Song, K. et al. Heart repair by reprogramming non-myocytes with cardiac transcription factors. Nature 485, 599–604 (2012).
Qian, L. et al. In vivo reprogramming of murine cardiac fibroblasts into induced cardiomyocytes. Nature 485, 593–598 (2012).
Torper, O. et al. Generation of induced neurons via direct conversion in vivo. Proc. Natl Acad. Sci. USA 110, 7038–7043 (2013).
Robel, S., Berninger, B. & Gotz, M. The stem cell potential of glia: lessons from reactive gliosis. Nat. Rev. Neurosci. 12, 88–104 (2011).
Sofroniew, M. V. & Vinters, H. V. Astrocytes: biology and pathology. Acta Neuropathol. 119, 7–35 (2010).
Sofroniew, M. V. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 32, 638–647 (2009).
Corti, S. et al. Direct reprogramming of human astrocytes into neural stem cells and neurons. Exp. Cell Res. 318, 1528–1541 (2012).
Lee, Y., Messing, A., Su, M. & Brenner, M. GFAP promoter elements required for region-specific and astrocyte-specific expression. Glia 56, 481–493 (2008).
Scott, C. E. et al. SOX9 induces and maintains neural stem cells. Nat. Neurosci. 13, 1181–1189 (2010).
Kuo, C. T. et al. Postnatal deletion of Numb/Numblike reveals repair and remodeling capacity in the subventricular neurogenic niche. Cell 127, 1253–1264 (2006).
Zhuo, L. et al. hGFAP-cre transgenic mice for manipulation of glial and neuronal function in vivo. Genesis 31, 85–94 (2001).
Gregorian, C. et al. Pten deletion in adult neural stem/progenitor cells enhances constitutive neurogenesis. J. Neurosci. 29, 1874–1886 (2009).
Bachoo, R. M. et al. Molecular diversity of astrocytes with implications for neurological disorders. Proc. Natl Acad. Sci. USA 101, 8384–8389 (2004).
Dutta, G., Barber, D. S., Zhang, P., Doperalski, N. J. & Liu, B. Involvement of dopaminergic neuronal cystatin C in neuronal injury-induced microglial activation and neurotoxicity. J. Neurochem. 122, 752–763 (2012).
Aronica, E. et al. Cystatin C, a cysteine protease inhibitor, is persistently up-regulated in neurons and glia in a rat model for mesial temporal lobe epilepsy. Eur. J. Neurosci. 14, 1485–1491 (2001).
Yokota, O. et al. Amyotrophic lateral sclerosis with dementia: an autopsy case showing many Bunina bodies, tau-positive neuronal and astrocytic plaque-like pathologies, and pallido-nigral degeneration. Acta Neuropathol. 112, 633–645 (2006).
Kettenmann, H., Hanisch, U. K., Noda, M. & Verkhratsky, A. Physiology of microglia. Physiol. Rev. 91, 461–553 (2011).
McKhann, G. M. 2nd, D’Ambrosio, R. & Janigro, D. Heterogeneity of astrocyte resting membrane potentials and intercellular coupling revealed by whole-cell and gramicidin-perforated patch recordings from cultured neocortical and hippocampal slice astrocytes. J. Neurosci. 17, 6850–6863 (1997).
Ge, W. P., Miyawaki, A., Gage, F. H., Jan, Y. N. & Jan, L. Y. Local generation of glia is a major astrocyte source in postnatal cortex. Nature 484, 376–380 (2012).
Richardson, W. D., Young, K. M., Tripathi, R. B. & McKenzie, I. NG2-glia as multipotent neural stem cells: fact or fantasy? Neuron 70, 661–673 (2011).
Zhu, X., Bergles, D. E. & Nishiyama, A. NG2 cells generate both oligodendrocytes and gray matter astrocytes. Development 135, 145–157 (2008).
Weber, P., Metzger, D. & Chambon, P. Temporally controlled targeted somatic mutagenesis in the mouse brain. Eur. J. Neurosci. 14, 1777–1783 (2001).
Hawasli, A. H. et al. Cyclin-dependent kinase 5 governs learning and synaptic plasticity via control of NMDAR degradation. Nat. Neurosci. 10, 880–886 (2007).
Madisen, L. et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 13, 133–140 (2010).
Chmielnicki, E., Benraiss, A., Economides, A. N. & Goldman, S. A. Adenovirally expressed noggin and brain-derived neurotrophic factor cooperate to induce new medium spiny neurons from resident progenitor cells in the adult striatal ventricular zone. J. Neurosci. 24, 2133–2142 (2004).
Cho, S. R. et al. Induction of neostriatal neurogenesis slows disease progression in a transgenic murine model of Huntington disease. J. Clin. Invest. 117, 2889–2902 (2007).
Hsieh, J., Nakashima, K., Kuwabara, T., Mejia, E. & Gage, F. H. Histone deacetylase inhibition-mediated neuronal differentiation of multipotent adult neural progenitor cells. Proc. Natl Acad. Sci. USA 101, 16659–16664 (2004).
Hao, Y. et al. Mood stabilizer valproate promotes ERK pathway-dependent cortical neuronal growth and neurogenesis. J. Neurosci. 24, 6590–6599 (2004).
Kreitzer, A. C. Physiology and pharmacology of striatal neurons. Annu. Rev. Neurosci. 32, 127–147 (2009).
Tepper, J. M., Tecuapetla, F., Koos, T. & Ibanez-Sandoval, O. Heterogeneity and diversity of striatal GABAergic interneurons. Front. Neuroanat. 4, 150 (2010).
Chew, L. J. & Gallo, V. The Yin and Yang of Sox proteins: Activation and repression in development and disease. J. Neurosci. Res. 87, 3277–3287 (2009).
Graham, V., Khudyakov, J., Ellis, P. & Pevny, L. SOX2 functions to maintain neural progenitor identity. Neuron 39, 749–765 (2003).
Ferri, A. L. et al. Sox2 deficiency causes neurodegeneration and impaired neurogenesis in the adult mouse brain. Development 131, 3805–3819 (2004).
Miyagi, S. et al. Consequence of the loss of Sox2 in the developing brain of the mouse. FEBS Lett. 582, 2811–2815 (2008).
Favaro, R. et al. Hippocampal development and neural stem cell maintenance require Sox2-dependent regulation of Shh. Nat. Neurosci. 12, 1248–1256 (2009).
Bani-Yaghoub, M. et al. Role of Sox2 in the development of the mouse neocortex. Dev. Biol. 295, 52–66 (2006).
Huang, E. J. & Reichardt, L. F. Neurotrophins: roles in neuronal development and function. Annu. Rev. Neurosci. 24, 677–736 (2001).
Altar, C. A. et al. Anterograde transport of brain-derived neurotrophic factor and its role in the brain. Nature 389, 856–860 (1997).
Lim, D. A. et al. Noggin antagonizes BMP signaling to create a niche for adult neurogenesis. Neuron 28, 713–726 (2000).
Fukumoto, T., Morinobu, S., Okamoto, Y., Kagaya, A. & Yamawaki, S. Chronic lithium treatment increases the expression of brain-derived neurotrophic factor in the rat brain. Psychopharmacology (Berl) 158, 100–106 (2001).
Hall, A. C. et al. Valproate regulates GSK-3-mediated axonal remodeling and synapsin I clustering in developing neurons. Mol. Cell Neurosci. 20, 257–270 (2002).
Srinivas, S. et al. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev. Biol. 1, 4 (2001).
Niu, W., Zou, Y., Shen, C. & Zhang, C. L. Activation of postnatal neural stem cells requires nuclear receptor TLX. J. Neurosci. 31, 13816–13828 (2011).
Wang, T. W. et al. Sox3 expression identifies neural progenitors in persistent neonatal and adult mouse forebrain germinative zones. J. Comp. Neurol. 497, 88–100 (2006).
Acknowledgements
We thank members of the Zhang laboratory for discussions and reagents. We also thank J. Bibb (UT Southwestern, USA) and P. Chambon (I.G.B.M.C., France) for providing PrP–CreERT mice, J. Hsieh (UT Southwestern, USA), C. Kuo (Duke University, USA) and Y. Jan (UCSF, USA) for Nes–CreERTM mice, M. Klymkowsky (UC Boulder, USA) for SOX3 antibody, K. Huber for sharing equipment, and E. Olson for critical reading of the manuscript. C-L.Z. is a W. W. Caruth, Jr. Scholar in Biomedical Research. This work was supported by The American Heart Association (09SDG2260602), The Whitehall Foundation Award (2009-12-05), The Welch Foundation Award (I-1724), The Ellison Medical Foundation Award (AG-NS-0753-11), and NIH Grants (1DP2OD006484 and R01NS070981; to C-L.Z.).
Author information
Authors and Affiliations
Contributions
W.N., T.Z. and C-L.Z. conceived and designed the experiments. W.N., T.Z., Y.Z. and C-L.Z. performed experiments. S.F. contributed to partial surgical experiments. D.K.S. critically reviewed and edited the manuscript. R.B. developed the Cst3–CreERT2 transgenic mice. W.N., T.Z. and C-L.Z. analysed data and prepared the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
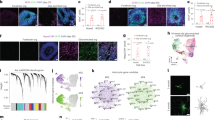
Supplementary Figure 1 The hGFAP promoter in lentivirus drives gene expression predominantly in astrocytes.
(a-d) Immunohistological analyses of GFP-expressing cells in striatal regions injected with hGFAP-GFP lentivirus. Arrows indicate co-labeled cells with the indicated markers. Scale: 20 μm. (e) Quantification of marker expression in GFP+ cells. Data are presented as mean+s.d. Mean is shown for n = 3 animals, scoring for each animal 250 or more GFP+ cells from 3-6 brain sections for each marker. n.d., not detected.
Supplementary Figure 2 Continually high expression of ectopic SOX2 prohibits robust induction of iANBs.
(a) Experimental design. Adult mice were injected with the indicated lentivirus. Gene expression was mediated by an hGFAP promoter or a constitutively active CAG promoter. Mice were analyzed 5 weeks later. (b) SOX2 expression in lentivirus-injected mouse brains. (c-d) DCX+ cells with extended neuronal processes are robustly detected in regions injected with hGFAP-SOX2 but not CAG-SOX2 lentivirus. Data are presented as mean+s.d., n = 4 animals per group. (e) SOX2 expression in DCX+ cells at the indicated weeks of post injection (wpi). The expression of SOX2 in DCX+ cells is greatly reduced at later stages in mice injected with virus expressing hGFAP-SOX2 but not CAG-SOX2. DCX+ cells are rarely observed from 1-3 wpi. Scales: 20 μm.
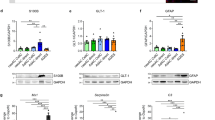
Supplementary Figure 3 Whole cell patch clamp parameters of traced cells in the striatum of Cst3-CreERT2;Rosa-tdT mice.
With the exception of one recorded cell has electrophysiological properties similar to microglia (indicated by an arrow), the remaining cells resemble astrocytes. V0, resting membrane potential; Rin, input resistance.
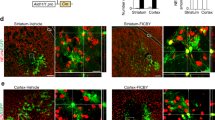
Supplementary Figure 4 Proliferating cells in the adult striatum pre- and post-lesions.
(a) Experimental design. Proliferating cells were labeled by BrdU administered with three intraperitoneal injections over a 6 h period before sacrifice. Striatal lesions were stereotactically introduced by a 30-gauge needle. hpl, hr post lesion; dpl, days post lesion. (b) Quantification of BrdU+ cells within the striatum. Very few proliferating cells were detected in the adult striatum before 3 dpl. Data are presented as mean+s.d., n = 4 lesion sites at each time point. (c) Distribution of proliferating cells among the examined cell types at 3 and 7 dpl. (d-e) Representative images showing labeled cells. Scales: 20 μm (d) and 50 μm (e)
Supplementary Figure 5 iANBs rarely generate mature neurons under normal conditions.
(a) Experimental design to examine newly generated mature neurons induced by SOX2. BrdU was administered in drinking water continuously for 4 weeks. (b-c) Quantification of BrdU-labeled DCX+ (b) or NeuN+ (c) cells in infected striatal regions. Data are presented as mean+s.d., n = 3 animals per group. (d) Immunofluorescence analysis showing a single BrdU+NeuN+ cell. Scale: 20 μm.
Supplementary Figure 6 Confocal images (a and e) and electrophysiological properties (b-d and f-h) of additional recorded tdT+ cells (indicated by arrows), which were loaded with biocytin (Bio) during recording.
Enlarged views of the boxed regions show detailed dendritic morphology of the recorded cells. (b-d) Electrophysiology of an aspiny tdT+ cells labeled in panel (a). (f-h) Electrophysiology of an aspiny tdT+ cells labeled in panel (e). These two cells fired repetitive action potentials in response to current step (b, f), exhibited inward currents in response to voltage step (c, g), and showed depolarizing spontaneous synaptic currents at resting membrane potential (d, h). Scales: 20 μm.
Supplementary Figure 7 Electrophysiological properties of SOX2-induced neurons.
Action potentials (left panels), inward currents upon depolarization (middle panels), and spontaneous synaptic currents (right panels) are shown for all 18 reprogrammed cells, which were traced in Cst3-CreERT2;Rosa-tdT mice. The three action potential traces shown were elicited by subthreshold current injection (bottom), just-suprathreshold depolarizing current injection (middle), and a more depolarizing current injection to generate the most frequent firing (top). The corresponding current injected was labeled besides the trace.
Supplementary Figure 8 Whole cell patch clamp parameters of SOX2-induced neurons with multiple action potentials.
V0, resting membrane potential; Rin, input resistance; C, capacitance; AP freq, frequency of action potentials.
Supplementary information
Supplementary Information
Supplementary Information (PDF 7995 kb)
Rights and permissions
About this article
Cite this article
Niu, W., Zang, T., Zou, Y. et al. In vivo reprogramming of astrocytes to neuroblasts in the adult brain. Nat Cell Biol 15, 1164–1175 (2013). https://doi.org/10.1038/ncb2843
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb2843
This article is cited by
-
Heimdall, an alternative protein issued from a ncRNA related to kappa light chain variable region of immunoglobulins from astrocytes: a new player in neural proteome
Cell Death & Disease (2023)
-
Temporal-spatial Generation of Astrocytes in the Developing Diencephalon
Neuroscience Bulletin (2023)
-
Generation of neural organoids for spinal-cord regeneration via the direct reprogramming of human astrocytes
Nature Biomedical Engineering (2022)
-
IRES-mediated Wnt2 translation in apoptotic neurons triggers astrocyte dedifferentiation
npj Regenerative Medicine (2022)
-
Induction of Nanog in neural progenitor cells for adaptive regeneration of ischemic brain
Experimental & Molecular Medicine (2022)