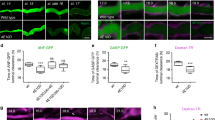
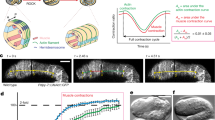
Abstract
Although many organ functions rely on epithelial tubes with correct dimensions, mechanisms underlying tube size control are poorly understood. We analyse the cellular mechanism of tracheal tube elongation in Drosophila, and describe an essential role of the conserved tyrosine kinase Src42A in this process. We show that Src42A is required for polarized cell shape changes and cell rearrangements that mediate tube elongation. In contrast, diametric expansion is controlled by apical secretion independently of Src42A. Constitutive activation of Src42A induces axial cell stretching and tracheal overelongation, indicating that Src42A acts instructively in this process. We propose that Src42A-dependent recycling of E-Cadherin at adherens junctions is limiting for cell shape changes and rearrangements in the axial dimension of the tube. Thus, we define distinct cellular processes that independently control axial and diametric expansion of a cylindrical epithelium in a developing organ. Whereas exocytosis-dependent membrane growth drives circumferential tube expansion, Src42A is required to orient membrane growth in the axial dimension of the tube.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Datta, A., Bryant, D. M. & Mostov, K. E. Molecular regulation of lumen morphogenesis. Curr. Biol. 21, R126–R136 (2011).
Lubarsky, B. & Krasnow, M. A. Tube morphogenesis: making and shaping biological tubes. Cell 112, 19–28 (2003).
Ghabrial, A., Luschnig, S., Metzstein, M. M. & Krasnow, M. A. Branching morphogenesis of the Drosophila tracheal system. Annu. Rev. Cell Dev. Biol. 19, 623–647 (2003).
Beitel, G. & Krasnow, M. Genetic control of epithelial tube size in the Drosophila tracheal system. Development 127, 3271–3282 (2000).
Förster, D., Armbruster, K. & Luschnig, S. Sec24-dependent secretion drives cell-autonomous expansion of tracheal tubes in Drosophila. Curr. Biol. 20, 62–68 (2010).
Tsarouhas, V. et al. Sequential pulses of apical epithelial secretion and endocytosis drive airway maturation in Drosophila. Dev. Cell 13, 214–225 (2007).
Devine, W. P. et al. Requirement for chitin biosynthesis in epithelial tube morphogenesis. Proc. Natl Acad. Sci. USA 102, 17014–17019 (2005).
Luschnig, S., Bätz, T., Armbruster, K. & Krasnow, M. serpentine and vermiform encode matrix proteins with chitin binding and deacetylation domains that limit tracheal tube length in Drosophila. Curr. Biol. 16, 186–194 (2006).
Tonning, A. et al. A transient luminal chitinous matrix is required to model epithelial tube diameter in the Drosophila trachea. Dev. Cell 9, 423–430 (2005).
Wang, S. et al. Septate-junction-dependent luminal deposition of chitin deacetylases restricts tube elongation in the Drosophila trachea. Curr. Biol. 16, 180–185 (2006).
Shindo, M. et al. Dual function of Src in the maintenance of adherens junctions during tracheal epithelial morphogenesis. Development 135, 1355–1364 (2008).
Takahashi, F., Endo, S., Kojima, T. & Saigo, K. Regulation of cell–cell contacts in developing Drosophila eyes by Dsrc41, a new, close relative of vertebrate c-src. Genes Dev. 10, 1645–1656 (1996).
Takahashi, M., Takahashi, F., Ui-Tei, K., Kojima, T. & Saigo, K. Requirements of genetic interactions between Src42A, armadillo and shotgun, a gene encoding E-cadherin, for normal development in Drosophila. Development 132, 2547–2559 (2005).
Tateno, M., Nishida, Y. & Adachi-Yamada, T. Regulation of JNK by Src during Drosophila development. Science 287, 324–327 (2000).
Matusek, T. et al. The Drosophila formin DAAM regulates the tracheal cuticle pattern through organizing the actin cytoskeleton. Development 133, 957–966 (2006).
Chung, S. et al. Serrano (sano) functions with the planar cell polarity genes to control tracheal tube length. PLoS Genet. 5, e1000746 (2009).
Usui, T. et al. Flamingo, a seven-pass transmembrane cadherin, regulates planar cell polarity under the control of Frizzled. Cell 98, 585–595 (1999).
Strutt, D., Johnson, R., Cooper, K. & Bray, S. Asymmetric localization of frizzled and the determination of notch-dependent cell fate in the Drosophila eye. Curr. Biol. 12, 813–824 (2002).
Strutt, D. I. Asymmetric localization of frizzled and the establishment of cell polarity in the Drosophila wing. Mol. Cell 7, 367–375 (2001).
Livet, J. et al. Transgenic strategies for combinatorial expression of fluorescent proteins in the nervous system. Nature 450, 56–62 (2007).
Martin-Blanco, E. et al. puckered encodes a phosphatase that mediates a feedback loop regulating JNK activity during dorsal closure in Drosophila. Genes Dev. 12, 557–570 (1998).
Rauzi, M., Lenne, P. F. & Lecuit, T. Planar polarized actomyosin contractile flows control epithelial junction remodelling. Nature 468, 1110–1114 (2010).
Choi, W. et al. The single Drosophila ZO-1 protein Polychaetoid regulates embryonic morphogenesis in coordination with Canoe/Afadin and Enabled. Mol. Biol. Cell 22, 2010–2030 (2011).
Fujita, Y. et al. Hakai, a c-Cbl-like protein, ubiquitinates and induces endocytosis of the E-cadherin complex. Nat. Cell Biol. 4, 222–231 (2002).
Huang, J., Zhou, W., Dong, W., Watson, A. M. & Hong, Y. Directed, efficient, and versatile modifications of the Drosophila genome by genomic engineering. Proc. Natl Acad. Sci. USA 106, 8284–8289 (2009).
Grieder, N. C. et al. γCOP is required for apical protein secretion and epithelial morphogenesis in Drosophila melanogaster. PLoS One 3, e3241 (2008).
Gumbiner, B. M. Regulation of cadherin-mediated adhesion in morphogenesis. Nat. Rev. Mol. Cell Biol. 6, 622–634 (2005).
Levina, E. M., Domnina, L. V., Rovensky, Y. A. & Vasiliev, J. M. Cylindrical substratum induces different patterns of actin microfilament bundles in nontransformed and in ras-transformed epitheliocytes. Exp. Cell Res. 229, 159–165 (1996).
Han, B. et al. Conversion of mechanical force into biochemical signaling. J. Biol. Chem 279, 54793–54801 (2004).
Wang, Y. et al. Visualizing the mechanical activation of Src. Nature 434, 1040–1045 (2005).
Ribeiro, C., Neumann, M. & Affolter, M. Genetic control of cell intercalation during tracheal morphogenesis in Drosophila. Curr. Biol. 14, 2197–2207 (2004).
Shaye, D. D., Casanova, J. & Llimargas, M. Modulation of intracellular trafficking regulates cell intercalation in the Drosophila trachea. Nat. Cell Biol. 10, 964–970 (2008).
Caussinus, E., Colombelli, J. & Affolter, M. Tip-cell migration controls stalk-cell intercalation during Drosophila tracheal tube elongation. Curr. Biol. 18, 1727–1734 (2008).
Sweeney, W. E., von Vigier, R. O., Frost, P. & Avner, E. D. Src inhibition ameliorates polycystic kidney disease. J. Am. Soc. Nephrol. 19, 1331–1341 (2008).
Oda, H. & Tsukita, S. Dynamic features of adherens junctions during Drosophila embryonic epithelial morphogenesis revealed by a Dα-catenin–GFP fusion protein. Dev. Genes Evol. 209, 218–225 (1999).
Pedraza, L. G., Stewart, R. A., Li, D-M. & Xu, T. Drosophila Src-family kinases function with Csk to regulate cell proliferation and apoptosis. Oncogene 23, 4754–4762 (2004).
Buszczak, M. et al. The Carnegie protein trap library: a versatile tool for Drosophila developmental studies. Genetics 175, 1505–1531 (2007).
Shiga, Y., Tanaka-Matakatsu, M. & Hayashi, S. A nuclear GFP/β-galactosidase fusion protein as a marker for morphogenesis in living Drosophila. Dev. Growth Diffn. 38, 99–106 (1996).
Zhang, L. & Ward, R. E. t. Uninflatable encodes a novel ectodermal apical surface protein required for tracheal inflation in Drosophila. Dev. Biol. 336, 201–212 (2009).
Aubouy, M., Jiang, Y., Glazier, J. A. & Graner, F. A texture tensor to quantify deformations. Granular Matter 5, 67–70 (2003).
Acknowledgements
We are indebted to K. Armbruster and S. Limmer, who isolated the Src42A mutants, and to T. Aegerter-Wilmsen for help with MATLAB analyses. We thank R. Güttinger for help with cell tracking, T. Aegerter-Wilmsen, C. Aegerter, M. Gonzalez-Gaitan, J. Grosshans, R. Metzger and D. Strutt for advice and comments on the manuscript and C. Lehner for support and discussions. We thank M. Affolter, K. Basler, E. Caussinus, J. Grosshans, S. Hayashi, Y. Hong, T. Kojima, T. Lecuit, A. Müller, D. Strutt, R. Ward and T. Xu for providing antibodies and fly stocks and J. Livet for providing Brainbow plasmids. We apologize to colleagues whose work could not be cited owing to space limitations. This work was supported by the German Research Foundation (DFG LU-1398/1-1), the Swiss National Science Foundation (SNF 3100A0_120713), the RPH-Promotor Stiftung, the Julius Klaus-Stiftung Zürich, the University of Zürich and the Kanton Zürich. D.F. was supported by a Müller fellowship of the Zürich Ph.D. programme in Molecular Life Sciences and by the Forschungskredit of the University of Zürich.
Author information
Authors and Affiliations
Contributions
D.F. and S.L. designed the experiments, D.F. carried out the experiments, D.F. and S.L. analysed the data. D.F. and S.L. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 1418 kb)
Supplementary Movie 1
Supplementary Information (MOV 9799 kb)
Supplementary Movie 2
Supplementary Information (MOV 7583 kb)
Supplementary Movie 3
Supplementary Information (MOV 10728 kb)
Supplementary Movie 4
Supplementary Information (MOV 11331 kb)
Rights and permissions
About this article
Cite this article
Förster, D., Luschnig, S. Src42A-dependent polarized cell shape changes mediate epithelial tube elongation in Drosophila. Nat Cell Biol 14, 526–534 (2012). https://doi.org/10.1038/ncb2456
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb2456
This article is cited by
-
An allosteric switch between the activation loop and a c-terminal palindromic phospho-motif controls c-Src function
Nature Communications (2023)
-
Temporal-spatial low shear stress induces heterogenous distribution of hematopoietic stem cell budding in zebrafish
Cellular and Molecular Life Sciences (2022)
-
Sex dependent effect of maternal e-nicotine on F1 Drosophila development and airways
Scientific Reports (2021)
-
Long-term in vivo imaging of Drosophila larvae
Nature Protocols (2020)
-
WASH phosphorylation balances endosomal versus cortical actin network integrities during epithelial morphogenesis
Nature Communications (2019)