Abstract
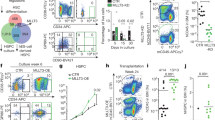
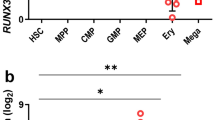
Successful haematopoiesis requires long-term retention of haematopoietic stem cells (HSCs) in a quiescent state. The transcriptional regulation of stem cell quiescence, especially by factors with specific functions in HSCs, is only beginning to be understood. Here, we demonstrate that Nurr1, a nuclear receptor transcription factor, has such a regulatory role. Overexpression of Nurr1 drives early haematopoietic progenitors into quiescence. When stem cells overexpressing Nurr1 are transplanted into lethally irradiated mice, they localize to the bone marrow, but do not contribute to regeneration of the blood system. Furthermore, the loss of only one allele of Nurr1 is sufficient to induce HSCs to enter the cell cycle and proliferate. Molecular analysis revealed an association between Nurr1 overexpression and upregulation of the cell-cycle inhibitor p18 (also known as INK4C), suggesting a mechanism by which Nurr1 could regulate HSC quiescence. Our findings provide critical insight into the transcriptional control mechanisms that determine whether HSCs remain dormant or enter the cell cycle and begin to proliferate.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Wilson, A. et al. Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell 135, 1118–1129 (2008).
Min, I. M. et al. The transcription factor EGR1 controls both the proliferation and localization of hematopoietic stem cells. Cell Stem Cell 2, 380–391 (2008).
Feng, C. G., Weksberg, D. C., Taylor, G. A., Sher, A. & Goodell, M. A. The p47 GTPase Lrg-47 (Irgm1) links host defense and hematopoietic stem cell proliferation. Cell Stem Cell 2, 83–89 (2008).
Liu, Y. et al. p53 regulates hematopoietic stem cell quiescence. Cell Stem Cell 4, 37–48 (2009).
Dykstra, B. et al. High-resolution video monitoring of hematopoietic stem cells cultured in single-cell arrays identifies new features of self-renewal. Proc. Natl Acad. Sci. USA 103, 8185–8190 (2006).
Cheng, T., Rodrigues, N., Dombkowski, D., Stier, S. & Scadden, D. T. Stem cell repopulation efficiency but not pool size is governed by p27(kip1). Nat. Med. 6, 1235–1240 (2000).
Cheng, T. et al. Hematopoietic stem cell quiescence maintained by p21cip1/waf1. Science 287, 1804–1808 (2000).
Kozar, K. et al. Mouse development and cell proliferation in the absence of D-cyclins. Cell 118, 477–491 (2004).
Malumbres, M. et al. Mammalian cells cycle without the D-type cyclin-dependent kinases Cdk4 and Cdk6. Cell 118, 493–504 (2004).
Viatour, P. et al. Hematopoietic stem cell quiescence is maintained by compound contributions of the retinoblastoma gene family. Cell Stem Cell 3, 416–428 (2008).
Ichikawa, M. et al. AML1/Runx1 negatively regulates quiescent hematopoietic stem cells in adult hematopoiesis. J. Immunol. 180, 4402–4408 (2008).
Hock, H. et al. Gfi-1 restricts proliferation and preserves functional integrity of haematopoietic stem cells. Nature 431, 1002–1007 (2004).
Venezia, T. A. et al. Molecular signatures of proliferation and quiescence in hematopoietic stem cells. PLoS Biol. 2, e301 (2004).
Chambers, S. M. et al. Hematopoietic fingerprints: an expression database of stem cells and their progeny. Cell Stem Cell 1, 578–591 (2007).
Maruyama, K. et al. The NGFI-B subfamily of the nuclear receptor superfamily (review). Int. J. Oncol. 12, 1237–1243 (1998).
Milbrandt, J. Nerve growth factor induces a gene homologous to the glucocorticoid receptor gene. Neuron 1, 183–188 (1988).
Ohkura, N., Hijikuro, M., Yamamoto, A. & Miki, K. Molecular cloning of a novel thyroid/steroid receptor superfamily gene from cultured rat neuronal cells. Biochem. Biophys. Res. Commun. 205, 1959–1965 (1994).
Law, S. W., Conneely, O. M., DeMayo, F. J. & O'Malley, B. W. Identification of a new brain-specific transcription factor, NURR1. Mol. Endocrinol. 6, 2129–2135 (1992).
Mullican, S. E. et al. Abrogation of nuclear receptors Nr4a3 and Nr4a1 leads to development of acute myeloid leukemia. Nat. Med. 13, 730–735 (2007).
Saucedo-Cardenas, O. et al. Nurr1 is essential for the induction of the dopaminergic phenotype and the survival of ventral mesencephalic late dopaminergic precursor neurons. Proc. Natl Acad. Sci. USA 95, 4013–4018 (1998).
Zetterstrom, R. H. et al. Dopamine neuron agenesis in Nurr1-deficient mice. Science 276, 248–250 (1997).
Ke, N. et al. Nuclear hormone receptor NR4A2 is involved in cell transformation and apoptosis. Cancer Res. 64, 8208–8212 (2004).
Castro, D. S. et al. Induction of cell cycle arrest and morphological differentiation by Nurr1 and retinoids in dopamine MN9D cells. J. Biol. Chem. 276, 43277–43284 (2001).
Sieburg, H. B. et al. The hematopoietic stem compartment consists of a limited number of discrete stem cell subsets. Blood 107, 2311–2316 (2006).
Camargo, F. D., Green, R., Capetanaki, Y., Jackson, K. A. & Goodell, M. A. Single hematopoietic stem cells generate skeletal muscle through myeloid intermediates. Nat. Med. 9, 1520–1527 (2003).
Nsegbe, E. et al. Congenital hypoventilation and impaired hypoxic response in Nurr1 mutant mice. J. Physiol. 556, 43–59 (2004).
Takeshita, M. et al. AML1–Evi-1 specifically transforms hematopoietic stem cells through fusion of the entire Evi-1 sequence to AML1. Leukemia 22, 1241–1249 (2008).
Kubota, Y., Osawa, M., Jakt, L. M., Yoshikawa, K. & Nishikawa, S. Necdin restricts proliferation of hematopoietic stem cells during hematopoietic regeneration. Blood 114, 4383–4392 (2009).
Wilson, T. E., Fahrner, T. J., Johnston, M. & Milbrandt, J. Identification of the DNA binding site for NGFI-B by genetic selection in yeast. Science 252, 1296–1300 (1991).
Maira, M., Martens, C., Philips, A. & Drouin, J. Heterodimerization between members of the Nur subfamily of orphan nuclear receptors as a novel mechanism for gene activation. Mol. Cell Biol. 19, 7549–7557 (1999).
Wilson, T. E., Fahrner, T. J. & Milbrandt, J. The orphan receptors NGFI-B and steroidogenic factor 1 establish monomer binding as a third paradigm of nuclear receptor–DNA interaction. Mol. Cell Biol. 13, 5794–5804 (1993).
Yuan, Y., Shen, H., Franklin, D. S., Scadden, D. T. & Cheng, T. In vivo self-renewing divisions of haematopoietic stem cells are increased in the absence of the early G1-phase inhibitor, p18INK4C. Nat. Cell Biol. 6, 436–442 (2004).
Wang, Y. Y. et al. Simultaneous knockdown of p18INK4C, p27Kip1 and MAD1 via RNA interference results in the expansion of long-term culture-initiating cells of murine bone marrow cells in vitro. Acta Biochim. Biophys. Sin. (Shanghai) 40, 711–720 (2008).
Yu, H., Yuan, Y., Shen, H. & Cheng, T. Hematopoietic stem cell exhaustion impacted by p18 INK4C and p21 Cip1/Waf1 in opposite manners. Blood 107, 1200–1206 (2006).
Saijo, K. et al. A Nurr1/CoREST pathway in microglia and astrocytes protects dopaminergic neurons from inflammation-induced death. Cell 137, 47–59 (2009).
Essers, M. A. et al. IFNα activates dormant haematopoietic stem cells in vivo. Nature 458, 904–908 (2009).
Martinat, C. et al. Cooperative transcription activation by Nurr1 and Pitx3 induces embryonic stem cell maturation to the midbrain dopamine neuron phenotype. Proc. Natl Acad. Sci. USA 103, 2874–2879 (2006).
Jacobs, F. M. et al. Pitx3 potentiates Nurr1 in dopamine neuron terminal differentiation through release of SMRT-mediated repression. Development 136, 531–540 (2009).
Lacorazza, H. D. et al. The transcription factor MEF/ELF4 regulates the quiescence of primitive hematopoietic cells. Cancer Cell 9, 175–187 (2006).
Ling, K. W. et al. GATA-2 plays two functionally distinct roles during the ontogeny of hematopoietic stem cells. J. Exp. Med. 200, 871–882 (2004).
Boyer, L. A. et al. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell 122, 947–956 (2005).
Naviaux, R. K., Costanzi, E., Haas, M. & Verma, I. M. The pCL vector system: rapid production of helper-free, high-titer, recombinant retroviruses. J. Virol. 70, 5701–5705 (1996).
Kotani, H. et al. Improved methods of retroviral vector transduction and production for gene therapy. Hum. Gene Ther. 5, 19–28 (1994).
Acknowledgements
We thank N. Boles, E. J. Dettman, and J. Gilbert for helpful discussion and suggestions, and T. Perlmann for comments on the manuscript. This work was supported by NIH grants DK58192, CA111411, CA126752, EB005173 and AG034451, as well as Ellison Foundation grant AG-SS178706, and the Dan L. Duncan Cancer Center. T. Cheng provided p18-null mice. We also thank S. Watowich for 32D cells.
Author information
Authors and Affiliations
Contributions
This study was developed and designed by O.S., who also performed the experiments and co-wrote the manuscript. G.L.L. and R.M. helped carry out the experiments. O.M.C. provided Nurr1−/− mice and discussion. M.A.G. designed experiments and co-wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 747 kb)
Rights and permissions
About this article
Cite this article
Sirin, O., Lukov, G., Mao, R. et al. The orphan nuclear receptor Nurr1 restricts the proliferation of haematopoietic stem cells. Nat Cell Biol 12, 1213–1219 (2010). https://doi.org/10.1038/ncb2125
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb2125
This article is cited by
-
Tespa1 facilitates hematopoietic and leukemic stem cell maintenance by restricting c-Myc degradation
Leukemia (2023)
-
CDK19 regulates the proliferation of hematopoietic stem cells and acute myeloid leukemia cells by suppressing p53-mediated transcription of p21
Leukemia (2022)
-
Drug targeting of NR4A nuclear receptors for treatment of acute myeloid leukemia
Leukemia (2019)
-
Single-cell analysis identifies a CD33+ subset of human cord blood cells with high regenerative potential
Nature Cell Biology (2018)
-
Impact of combinatorial dysfunctions of Tet2 and Ezh2 on the epigenome in the pathogenesis of myelodysplastic syndrome
Leukemia (2017)