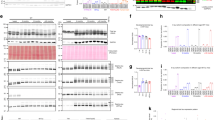
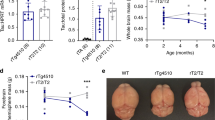
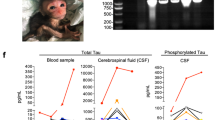
Abstract
Hyperphosphorylated tau makes up the filamentous intracellular inclusions of several neurodegenerative diseases, including Alzheimer's disease1. In the disease process, neuronal tau inclusions first appear in the transentorhinal cortex from where they seem to spread to the hippocampal formation and neocortex2. Cognitive impairment becomes manifest when inclusions reach the hippocampus, with abundant neocortical tau inclusions and extracellular β-amyloid deposits being the defining pathological hallmarks of Alzheimer's disease. An abundance of tau inclusions, in the absence of β-amyloid deposits, defines Pick's disease, progressive supranuclear palsy, corticobasal degeneration and other diseases1. Tau mutations cause familial forms of frontotemporal dementia, establishing that tau protein dysfunction is sufficient to cause neurodegeneration and dementia3,4,5. Thus, transgenic mice expressing mutant (for example, P301S) human tau in nerve cells show the essential features of tauopathies, including neurodegeneration and abundant filaments made of hyperphosphorylated tau protein6,8. By contrast, mouse lines expressing single isoforms of wild-type human tau do not produce tau filaments or show neurodegeneration7,8. Here we have used tau-expressing lines to investigate whether experimental tauopathy can be transmitted. We show that injection of brain extract from mutant P301S tau-expressing mice into the brain of transgenic wild-type tau-expressing animals induces assembly of wild-type human tau into filaments and spreading of pathology from the site of injection to neighbouring brain regions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Goedert, M. & Spillantini, M. G. A century of Alzheimer's disease. Science 314, 777–781 (2006).
Braak, H. & Braak, E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 82, 239–259 (1991).
Poorkaj, P. et al. Tau is a candidate gene for chromosome 17 frontotemporal dementia. Ann. Neurol. 43, 815–825 (1998).
Hutton, M. et al. Association of missense and 5´-splice-site mutations in tau with the inherited dementia FTDP-17. Nature 393, 702–705 (1998).
Spillantini, M. G. et al. Mutation in the tau gene in familial multiple system tauopathy with presenile dementia. Proc. Natl Acad. Sci. USA 95, 7737–7741 (1998).
Allen, B. et al. Abundant tau filaments and nonapoptotic neurodegeneration in transgenic mice expressing human P301S tau protein. J. Neurosci. 22, 9340–9351 (2002).
Probst, A. et al. Axonopathy and amyotrophy in mice transgenic for human four-repeat tau protein. Acta Neuropathol. 99, 469–481 (2000).
Frank, S., Clavaguera, F. & Tolnay, M. Tauopathy models and human neuropathology: similarities and differences. Acta Neuropathol. 115, 39–53 (2008).
Goedert, M., Spillantini, M. G., Jakes, R., Rutherford, D. & Crowther, R. A. Multiple isoforms of human microtubule-associated protein tau: Sequences and localization in neurofibrillary tangles of Alzheimer's disease. Neuron 3, 519–526 (1989).
Gallyas, F. Silver staining of Alzheimer's neurofibrillary changes by means of physical development. Acta Morphol. Acad. Sci. Hung. 19, 1–8 (1971).
Braak, H., Braak, E., Ohm, T. & Bohl, J. Silver impregnation of Alzheimer's neurofibrillary changes counterstained for basophilic material and lipofuscin pigment. Stain Technol. 63, 197–200 (1988).
Lee, V. M.-Y., Goedert, M. & Trojanowski, J. Q. Neurodegenerative tauopathies. Annu. Rev. Neurosci. 24, 1121–1159 (2001).
Crowther, R. A. & Goedert, M. Abnormal tau-containing filaments in neurodegenerative diseases. J. Struct. Biol. 130, 271–279 (2000).
Prusiner, S. B. Prions. Proc. Natl Acad. Sci. USA 95, 13363–13383 (1998).
Legname, G. et al. Synthetic mammalian prions. Science 305, 673–676 (2004).
Kane, M. D. et al. Evidence for seeding of β-amyloid by intracerebral infusion of Alzheimer brain extracts in β amyloid precursor protein-transgenic mice. J. Neurosci. 15, 3606–3611 (2000).
Meyer-Luehmann, M. et al. Exogenous induction of cerebral β-amyloidosis is governed by agent and host. Science 313, 1781–1784 (2006).
Götz, J., Chen, F., van Dorpe, J. & Nitsch, R. M. Formation of neurofibrillary tangles in P301L tau transgenic mice induced by Aβ 42 fibrils. Science 293, 1491–1495 (2001).
Bolmont, T. et al. Induction of tau pathology by intracerebral infusion of amyloid-β-containing brain extract and by amyloid-β deposition in APP X tau transgenic mice. Am. J. Pathol. 171, 2012–2020 (2007).
Xing, Y. et al. Transmission of mouse senile amyloidosis. Lab. Invest. 81, 493–499 (2001).
Lundmark, K. et al. Transmissibility of systemic amyloidosis by a prion-like mechanism. Proc. Natl Acad. Sci. USA 99, 6979–6984 (2002).
Walker, L. C., LeVine III, H., Mattson, M. P. & Jucker, M. Inducible proteopathies. Trends Neurosci. 29, 438–443 (2006).
Boutajangout. et al. Characterisation of cytoskeletal abnormalities in mice transgenic for wild-type human tau and familial Alzheimer's disease mutants of APP and presenilin-1. Neurobiol. Dis. 15, 47–60 (2004).
Urushitani, M. et al. Chromogranin-mediated secretion of mutant superoxide dismutase proteins linked to amyotrophic lateral sclerosis. Nature Neurosci. 9, 108–118 (2006).
Asuni, A. A., Boutajangout, A., Quartermain, D. & Sigurdson, E. M. Immunotherapy targeting pathological tau conformers in a tangle mouse model reduces brain pathology with associated functional improvements. J. Neurosci. 27, 9115–9129 (2007).
Braak, H. et al. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol. Aging 24, 197–211 (2003).
Brundin, P., Li, J.-Y., Holton, J. L., Lindvall, O. & Revesz, T. Research in motion: the enigma of Parkinson's disease pathology spread. Nature Rev. Neurosci. 9, 741–745 (2008).
Brown, P. et al. Human spongiform encephalopathy: The National Institutes of Health series of 300 cases of experimentally transmitted disease. Ann. Neurol. 35, 513–529 (1994).
Mercken, M. et al. Affinity purification of human tau proteins and the construction of a sensitive sandwich enzyme-linked immunosorbent assay for human tau detection. J. Neurochem. 58, 548–553 (1992).
Goedert, M., Jakes, R. & Vanmechelen, E. Monoclonal antibody AT8 recognises tau protein phosphorylated at both serine 202 and threonine 205. Neurosci. Lett. 189, 167–170 (1995).
Yoshida, H. & Goedert, M. Sequential phosphorylation of tau protein by cAMP-dependent protein kinase and SAPK4/p38δ or JNK2 in the presence of heparin generates the AT100 epitope. J. Neurochem. 99, 154–164 (2006).
Kosik, K. S. et al. Epitopes that span the tau molecule are shared with paired helical filaments. Neuron 1, 817–825 (1988).
Crowther, R. A. Straight and paired helical filaments in Alzheimer disease have a common structural unit. Proc. Natl Acad. Sci. USA 88, 2288–2292 (1991).
Goedert, M., Spillantini, M. G., Cairns, N. J. & Crowther, R. A. Tau proteins of Alzheimer paired helical filaments: Abnormal phosphorylation of all six brain isoforms. Neuron 8, 159–168 (1992).
Franklin, K.B. J. & Paxinos, G. The mouse brain in stereotaxic coordinates. (Academic Press, New York, 2001).
Acknowledgements
This work was supported by the Swiss National Science Foundation (3100AO-120261) (M.T.), the Alzheimer Association (ZEN-06-27341), the German National Genome Network (NGFN-Plus) and the German Competence Network in Degenerative Dementias (01GI0705) (M.J.), the U.K. Medical Research Council (R.A.C, G.F., M.G.) and the U.K. Alzheimer's Research Trust (M.G.). We thank K.H. Wiederhold (Novartis Institutes for Biomedical Research, Basel) and N. Schaeren-Wiemers (University Hospital Basel) for antibodies and helpful discussions.
Author information
Authors and Affiliations
Contributions
F.C., R.A.C., M.G. and M.T. designed the experiments, coordinated the project and wrote the manuscript. M.J. initiated the study. F.C., T.B., R.A.C. D.A., G.F., A.K.S. and M.G. performed the experimental work. A.P. assisted with assessment and interpretation of initiation and neuroanatomical spreading of tau pathology. M.B. performed statistical analyses. M.S., S.F. and M.J. contributed to data and manuscript discussions.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 902 kb)
Rights and permissions
About this article
Cite this article
Clavaguera, F., Bolmont, T., Crowther, R. et al. Transmission and spreading of tauopathy in transgenic mouse brain. Nat Cell Biol 11, 909–913 (2009). https://doi.org/10.1038/ncb1901
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1901
This article is cited by
-
Brain clearance of protein aggregates: a close-up on astrocytes
Molecular Neurodegeneration (2024)
-
Neuropathogenesis-on-chips for neurodegenerative diseases
Nature Communications (2024)
-
Native PLGA nanoparticles attenuate Aβ-seed induced tau aggregation under in vitro conditions: potential implication in Alzheimer’s disease pathology
Scientific Reports (2024)
-
Decoding the Cellular Trafficking of Prion-like Proteins in Neurodegenerative Diseases
Neuroscience Bulletin (2024)
-
Tau and neuroinflammation in Alzheimer’s disease: interplay mechanisms and clinical translation
Journal of Neuroinflammation (2023)