Abstract
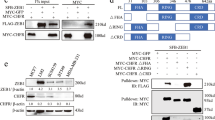
Chfr is a ubiquitin ligase that functions in the mitotic checkpoint by delaying entry into metaphase in response to mitotic stress1,2. It has been suggested that Chfr is a tumour suppressor as Chfr is frequently silenced in human cancers3. To better understand how Chfr activity relates to cell-cycle progression and tumorigenesis, we sought to identify Chfr-interacting proteins using affinity purification combined with mass spectrometry. Histone deacetylase 1 (HDAC1), which represses transcription by deacetylating histones, was newly isolated as a Chfr-interacting protein. Chfr binds and downregulates HDAC1 by inducing its polyubiquitylation, both in vitro and in vivo. Ectopic expression of Chfr in cancer cells that normally do not express it results in downregulation of HDAC1, leading to upregulation of the Cdk inhibitor p21CIP1/WAF1 and the metastasis suppressors KAI1 and E-cadherin. Coincident with these changes, cells arrest in the G1 phase of the cell cycle and become less invasive. Collectively, our data suggest that Chfr functions as a tumour suppressor by regulating HDAC1.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Kang, D., Chen, J., Wong, J. & Fang, G. The checkpoint protein Chfr is a ligase that ubiquitinates Plk1 and inhibits Cdc2 at the G2 to M transition. J. Cell Biol. 156, 249–259 (2002).
Yu, X. et al. Chfr is required for tumor suppression and Aurora A regulation. Nature Genet. 37, 401–406 (2005).
Scolnick, D. M. & Halazonetis, T. D. Chfr defines a mitotic stress checkpoint that delays entry into metaphase. Nature 406, 430–435 (2000).
Bothos, J., Summers, M. K., Venere, M., Scolnick, D. M. & Halazonetis, T. D. The Chfr mitotic checkpoint protein functions with Ubc13–Mms2 to form Lys 63-linked polyubiquitin chains. Oncogene 22, 7101–7107 (2003).
Chaturvedi, P. et al. Chfr regulates a mitotic stress pathway through its RING-finger domain with ubiquitin ligase activity. Cancer Res. 62, 1797–1801 (2002).
Oh, Y. M., Yoo, S. J. & Seol, J. H. Deubiquitination of Chfr, a checkpoint protein, by USP7/HAUSP regulates its stability and activity. Biochem. Biophys. Res. Commun. 357, 615–619 (2007).
Matsusaka, T. & Pines, J. Chfr acts with the p38 stress kinases to block entry to mitosis in mammalian cells. J. Cell Biol. 166, 507–516 (2004).
Thiagalingam, S. et al. Histone deacetylases: unique players in shaping the epigenetic histone code. Ann. NY Acad. Sci. 983, 84–100 (2003).
Tsai, M. D. FHA: a signal transduction domain with diverse specificity and function. Structure 10, 887–888 (2002).
Stavridi, E. S. et al. Crystal structure of the FHA domain of the Chfr mitotic checkpoint protein and its complex with tungstate. Structure 10, 891–899 (2002).
Wang, A. G. et al. Histone deacetylase 1 contributes to cell cycle and apoptosis. Biol. Pharm. Bull. 28, 1966–1970 (2005).
Kim, J. H. et al. Transcriptional regulation of a metastasis suppressor gene by Tip60 and β-catenin complexes. Nature 434, 921–926 (2005).
David, G., Neptune, M. A. & DePinho, R. A. SUMO-1 modification of histone deacetylase 1 (HDAC1) modulates its biological activities. J. Biol. Chem. 277, 23658–23663 (2002).
Ito, A. et al. MDM2-HDAC1-mediated deacetylation of p53 is required for its degradation. EMBO J. 21, 6236–6245 (2002).
Ocker, M. & Schneider-Stock, R. Histone deacetylase inhibitors: signalling towards p21cip1/waf1. Int. J. Biochem. Cell Biol. 39, 1367–1374 (2007).
Li, H. & Wu, X. Histone deacetylase inhibitor, trichostatin A, activates p21WAF1/CIP1 expression through downregulation of c-Myc and release of the repression of c-Myc from the promoter in human cervical cancer cells. Biochem. Biophys. Res. Commun. 324, 860–867 (2004).
Senese, S. et al. Role for histone deacetylase 1 in human tumor cell proliferation. Mol. Cell. Biol. 27, 4784–4795 (2007).
Mizuno, K. et al. Aberrant hypermethylation of the CHFR prophase checkpoint gene in human lung cancers. Oncogene 21, 2328–2333 (2002).
Brandes, J. C., van Engeland, M., Wouters, K. A., Weijenberg, M. P. & Herman, J. G. CHFR promoter hypermethylation in colon cancer correlates with the microsatellite instability phenotype. Carcinogenesis 26, 1152–1156 (2005).
Privette, L. M., Gonzalez, M. E., Ding, L., Kleer, C. G. & Petty, E. M. Altered expression of the early mitotic checkpoint protein, CHFR, in breast cancers: implications for tumor suppression. Cancer Res. 67, 6064–6074 (2007).
Cheng, C. W. et al. Mechanisms of inactivation of E-cadherin in breast carcinoma: modification of the two-hit hypothesis of tumor suppressor gene. Oncogene 20, 3814–3823 (2001).
Yang, J. Y. et al. MDM2 promotes cell motility and invasiveness by regulating E-cadherin degradation. Mol. Cell. Biol. 26, 7269–7282 (2006).
Kim, J. H. et al. Roles of sumoylation of a reptin chromatin-remodelling complex in cancer metastasis. Nature Cell Biol. 8, 631–639 (2006).
Baker, D. J., Chen, J. & van Deursen, J. M. The mitotic checkpoint in cancer and aging: what have mice taught us? Curr. Opin. Cell Biol. 17, 583–589 (2005).
Cao, R., Tsukada, Y. & Zhang, Y. Role of Bmi-1 and Ring21A in H2A ubiquitylation and Hox gene silencing. Mol. Cell 20, 845–854 (2005).
Acknowledgements
We thank S. H. Baek (SNU) for reagents. This work was supported by grants from the Korea Science and Engineering Foundation (M10533010001-07N3301-00110), the SRC program (R11-2005-009-02002-0), the Korea Research Foundation (KRF-2002-015-CS0069) and the BK21 program. Y.E.K. was supported by the Seoul Science Fellowship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 1264 kb)
Rights and permissions
About this article
Cite this article
Oh, Y., Kwon, Y., Kim, J. et al. Chfr is linked to tumour metastasis through the downregulation of HDAC1. Nat Cell Biol 11, 295–302 (2009). https://doi.org/10.1038/ncb1837
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1837
This article is cited by
-
Targeting the E2F1/Rb/HDAC1 axis with the small molecule HR488B effectively inhibits colorectal cancer growth
Cell Death & Disease (2023)
-
Childhood DNA methylation as a marker of early life rapid weight gain and subsequent overweight
Clinical Epigenetics (2021)
-
EGFR phosphorylates HDAC1 to regulate its expression and anti-apoptotic function
Cell Death & Disease (2021)
-
CHFR regulates chemoresistance in triple-negative breast cancer through destabilizing ZEB1
Cell Death & Disease (2021)
-
Marek’s disease virus Meq oncoprotein interacts with chicken HDAC 1 and 2 and mediates their degradation via proteasome dependent pathway
Scientific Reports (2021)