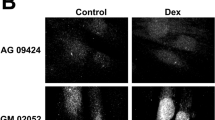
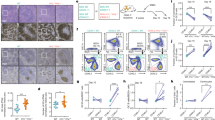
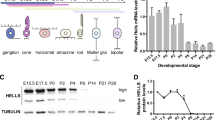
Abstract
Lamina-associated polypeptide (LAP) 2α is a chromatin-associated protein that binds A-type lamins1,2. Mutations in both LAP2α and A-type lamins are linked to human diseases called laminopathies3, but the molecular mechanisms are poorly understood. The A-type lamin–LAP2α complex interacts with and regulates retinoblastoma protein (pRb)4,5, but the significance of this interaction in vivo is unknown. Here we address the function of the A-type lamin–LAP2α complex with the use of LAP2α-deficient mice. We show that LAP2α loss causes relocalization of nucleoplasmic A-type lamins to the nuclear envelope and impairs pRb function. This causes inefficient cell-cycle arrest in dense fibroblast cultures and hyperproliferation of epidermal and erythroid progenitor cells in vivo, leading to tissue hyperplasia. Our results support a disease-relevant model6 in which LAP2α defines A-type lamin localization in the nucleoplasm, which in turn affects pRb-mediated regulation of progenitor cell proliferation and differentiation in highly regenerative tissues.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Dechat, T. et al. Nuclear lamins: major factors in the structural organization and function of the nucleus and chromatin. Genes Dev. 22, 832–853 (2008).
Schirmer, E. C. & Foisner, R. Proteins that associate with lamins: many faces, many functions. Exp. Cell Res. 313, 2167–2179 (2007).
Capell, B. C. & Collins, F. S. Human laminopathies: nuclei gone genetically awry. Nature Rev. Genet. 7, 940–952 (2006).
Dorner, D. et al. Lamina-associated polypeptide 2α regulates cell cycle progression and differentiation via the retinoblastoma–E2F pathway. J. Cell Biol. 173, 83–93 (2006).
Pekovic, V. et al. Nucleoplasmic LAP2α–lamin A complexes are required to maintain a proliferative state in human fibroblasts. J. Cell Biol. 176, 163–172 (2007).
Gotzmann, J. & Foisner, R. A-type lamin complexes and regenerative potential: a step towards understanding laminopathic diseases? Histochem. Cell Biol. 125, 33–41 (2006).
Berger, R. et al. The characterization and localization of the mouse thymopoietin/lamina-associated polypeptide 2 gene and its alternatively spliced products. Genome Res. 6, 361–370 (1996).
Dechat, T. et al. Lamina-associated polypeptide 2α binds intranuclear A-type lamins. J. Cell Sci. 19, 3473–3484 (2000).
Taylor, M. R. et al. Thymopoietin (lamina-associated polypeptide 2) gene mutation associated with dilated cardiomyopathy. Hum. Mutat. 26, 566–574 (2005).
Schwenk, F., Baron, U. & Rajewsky, K. A cre-transgenic mouse strain for the ubiquitous deletion of loxP-flanked gene segments including deletion in germ cells. Nucleic Acids Res. 23, 5080–5081 (1995).
Sage, J. et al. Targeted disruption of the three Rb-related genes leads to loss of G1 control and immortalization. Genes Dev. 14, 3037–3050 (2000).
Sankaran, V. G., Orkin, S. H. & Walkley, C. R. Rb intrinsically promotes erythropoiesis by coupling cell cycle exit with mitochondrial biogenesis. Genes Dev. 22, 463–475 (2008).
Spike, B. T. et al. The Rb tumor suppressor is required for stress erythropoiesis. EMBO J. 23, 4319–4329 (2004).
Socolovsky, M. et al. Ineffective erythropoiesis in Stat5a−/−5b−/− mice due to decreased survival of early erythroblasts. Blood 98, 3261–3273 (2001).
Iavarone, A. et al. Retinoblastoma promotes definitive erythropoiesis by repressing Id2 in fetal liver macrophages. Nature 432, 1040–1045 (2004).
Moir, R. D., Yoon, M., Khuon, S. & Goldman, R. D. Nuclear lamins A and B1: different pathways of assembly during nuclear envelope formation in living cells. J. Cell Biol. 151, 1155–1168 (2000).
Markiewicz, E., Ledran, M. & Hutchison, C. J. Remodelling of the nuclear lamina and nucleoskeleton is required for skeletal muscle differentiation in vitro. J. Cell Sci. 118, 409–420 (2005).
Walkley, C. R. & Orkin, S. H. Rb is dispensable for self-renewal and multilineage differentiation of adult hematopoietic stem cells. Proc. Natl Acad. Sci. USA 103, 9057–9062 (2006).
Ruiz, S. et al. Unique and overlapping functions of pRb and p107 in the control of proliferation and differentiation in epidermis. Development 131, 2737–2748 (2004).
Haigis, K., Sage, J., Glickman, J., Shafer, S. & Jacks, T. The related retinoblastoma (pRb) and p130 proteins cooperate to regulate homeostasis in the intestinal epithelium. J. Biol. Chem. 281, 638–647 (2006).
Janzen, V. et al. Stem-cell ageing modified by the cyclin-dependent kinase inhibitor p16INK4a. Nature 443, 421–426 (2006).
Molofsky, A. V. et al. Increasing p16INK4a expression decreases forebrain progenitors and neurogenesis during ageing. Nature 443, 448–452 (2006).
Krishnamurthy, J. et al. p16INK4a induces an age-dependent decline in islet regenerative potential. Nature 443, 453–457 (2006).
Constantinescu, D., Gray, H. L., Sammak, P. J., Schatten, G. P. & Csoka, A. B. Lamin A/C expression is a marker of mouse and human embryonic stem cell differentiation. Stem Cells 24, 177–185 (2006).
Pajerowski, J. D., Dahl, K. N., Zhong, F. L., Sammak, P. J. & Discher, D. E. Physical plasticity of the nucleus in stem cell differentiation. Proc. Natl Acad. Sci. USA 104, 15619–15624 (2007).
Espada, J. et al. Nuclear envelope defects cause stem cell dysfunction in premature-aging mice. J. Cell Biol. 181, 27–35 (2008).
Sagelius, H. et al. Targeted transgenic expression of the mutation causing Hutchinson–Gilford progeria syndrome leads to proliferative and degenerative epidermal disease. J. Cell. Sci. 121, 969–978 (2008).
Scaffidi, P. & Misteli, T. Lamin A-dependent misregulation of adult stem cells associated with accelerated ageing. Nature Cell Biol. 10, 452–459 (2008).
Andra, K., Nikolic, B., Stocher, M., Drenckhahn, D. & Wiche, G. Not just scaffolding: plectin regulates actin dynamics in cultured cells. Genes Dev. 12, 3442–3451 (1998).
Vlcek, S., Just, H., Dechat, T. & Foisner, R. Functional diversity of LAP2α and LAP2β in postmitotic chromosome association is caused by an α-specific nuclear targeting domain. EMBO J. 18, 6370–6384 (1999).
Dechat, T. et al. Detergent-salt resistance of LAP2α in interphase nuclei and phosphorylation-dependent association with chromosomes early in nuclear assembly implies functions in nuclear structure dynamics. EMBO J. 17, 4887–4902 (1998).
Vlcek, S., Korbei, B. & Foisner, R. Distinct functions of the unique C terminus of LAP2α in cell proliferation and nuclear assembly. J. Biol. Chem. 277, 18898–18907 (2002).
Loewinger, L. & McKeon, F. Mutations in the nuclear lamin proteins resulting in their aberrant assembly in the cytoplasm. EMBO J. 7, 2301–2309 (1988).
Acknowledgements
We thank W. Müller for the Cre-deleter mouse strain, Larry Gerace for the genomic BAC clone, T. Jacks for triple knockout mouse embryonic fibroblasts, P. Luvalle for providing paws from lamin A/C-deficient mice, K. Biadasiewicz for expert technical assistance, and H. Beug and E. W. Müllner for helpful discussions. This study was supported by grants from the Austrian Science Research Fund (FWF P17871) and the EURO-Laminopathies research project of the European Commission (contract LSHM-CT-2005-018690) to R.F. and a postdoctoral fellowship from L'Oreal/UNESCO/ÖADW/BMWF to N.N.
Author information
Authors and Affiliations
Contributions
N.N., B.K., P.F., C.L.S. and R.F. planned the project; N.N., B.K., S.K., M.A.K., D.D., R.K., I.G. and T.C. performed the experiments; N.N., B.K., M.A.K., D.D., R.B. and R.F. analysed the data; N.N., B.K., C.L.S. and R.F. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 1068 kb)
Rights and permissions
About this article
Cite this article
Naetar, N., Korbei, B., Kozlov, S. et al. Loss of nucleoplasmic LAP2α–lamin A complexes causes erythroid and epidermal progenitor hyperproliferation. Nat Cell Biol 10, 1341–1348 (2008). https://doi.org/10.1038/ncb1793
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1793
This article is cited by
-
LAP2α preserves genome integrity through assisting RPA deposition on damaged chromatin
Genome Biology (2022)
-
PCA3 controls chromatin organization and p53 signal activation by regulating LAP2α-lamin A complexes
Cancer Gene Therapy (2022)
-
Lamina-associated polypeptide 2α is required for intranuclear MRTF-A activity
Scientific Reports (2022)
-
Integrated analysis reveals the alterations that LMNA interacts with euchromatin in LMNA mutation-associated dilated cardiomyopathy
Clinical Epigenetics (2021)
-
The nucleoplasmic interactions among Lamin A/C-pRB-LAP2α-E2F1 are modulated by dexamethasone
Scientific Reports (2021)